Why pure water is very weak electrolyte?
a) It ionizes to a large extent.
b) It ionizes to very small extent.
c) It does not ionizes at all.
d) It ionizes completely.
5) Water molecule is a highly polar solvent due to:
a) Presence of two lone pair of electrons.
b) Presence of three lone pair of electrons.
c) Absence of lone pair of electrons.
d) None of these.
LABORATORY WORK
REDOX REACTIONS
Objective: to investigate the redox reactions and chemical properties of main oxidants.
Reactions involving oxidation and reduction processes are very important in our everyday world. They make batteries work and cause metals to corrode (or help to prevent their corrosion). They enable us to obtain heat by burning fuels in factories and in our bodies.
Oxidation-Reduction Reactions are all reactions that involve the change of an oxidation number, and transfer of electrons among the reacting substances.
Oxidation is the loss of electrons. When a species loses electrons it is said to be oxidized:
Fe3+ – 1e àFe2+
Reduction is the gain of electrons. When a species gains electrons it is said to be reduced:
MnO4- + 8H+ + 5e à Mn2+ + 4H2O
Overall redox equations can be created by combining the half-equations for the oxidation process and reduction processes, after multiplying all the coefficients of the species in one of the half-equations by a factor which ensures that the number of electrons gained is equal to the number of electrons lost.
Fe3+ – 1e à Fe2+ 1 5 oxidation
MnO4- + 8H+ + 5e à Mn2+ + 4H2O 5 1 reduction
Multiplying all coefficients in the oxidation reaction by 5:
5Fe3+ – 5e à 5Fe2+
means that 5 electrons are gained and five are lost
and overall equation:
MnO4- + 8H+ + 5Fe2+ à Mn2+ + 4H2O + 5Fe3+
A species which can accept (+ne) electrons from another species is an oxidising agent (Cl2, KMnO4, K2Cr2O7, HNO3 concentrated, H2O2). Oxidising agents are reduced during redox reactions. MnO4- is the oxidizing agent in the above reaction.
A species which can donate (–ne) electrons to another species is a reducing agent (metals, SO2, H2S gas, Na2SO3, SnCl2 solution). Reducing agents are oxidised during redox reactions. Fe2+ is the reducing agent in the above reaction.
|
|
|
The main property of oxidising agent and reducing agent are their equivalent weight (mass) that react with one mole of electrons in a redox reaction:

Types of Redox reactions
1) Single-Replacement Redox Reactions: A + BC ® AC + B
• Conventional (Molecular) Equation
Zn(s) + Cu(NO3)2 (aq) → Zn(NO3)2(aq) + Cu(s)
• Ionic Equation
Zn(s) + Cu+2(aq) + NO3- (aq) → Zn+2(aq) + NO3- (aq) + Cu(s)
• Net Ionic Equation
Zn(s) + Cu+2(aq) → Zn+2(aq) + Cu(s)
2) Combination reaction is a reaction in which two or more substances are combined to form a single product: A +B + C ® ABC
C (s) + O2 (g) → CO2 (g)
3) Decomposition reaction is a reaction in which a single compound reacts to give two or more substances, usually requiring a raise in temperature:
ABC ® A + B + C
2KClO3 (s) ® 2KCl (s) + 3O2 (g)
4) Combustion reaction is a reaction of a substance with oxygen, usually the rapid release of heat produces a flame:
CH4 (g) + 2O2 (g) ® CO2 (g) + 2H2O (g)
5) Disproportionation reaction is a intramolecular reaction in which the atoms of one element is reduced and simultaneously increase the degree of oxidation state.
+1 -1 +1 -2 0
H2O2 ® H2O + O2
To predict the products of a redox reaction, look at the reagents given to see if there is both an oxidizing agent and a reducing agent. When a problem mentions an acidic or basic solution, it is probably redox.
| Common oxidizing agents | Products formed |
| MnO4- in acidic solution MnO2 in acidic solution MnO4- in neutral or basic solution Cr2O7- in acidic solution HNO3, concentrated HNO3, dilute H2SO4, hot, concentrated metalic ions free halogens Na2O2 HC1O4 H2O2 | Mn2+ Mn2+ MnO2 (s) Cr3+ NO2 NO SO2 metalous ions halide ions NaOH Cl- H2O |
| Common reducing agents | Products formed |
| halide ions free metals sulfite ions (SO32-) or SO2 nitrite ions (NO2-) free halogens, dilute basic solution free halogens, conc. basic solution metalous ions H2O2 C2O42- | free halogen metal ions sulfate ions (SO42-) nitrate ions (NO3-) hypohalite ions halate ions metalic ions O2 CO2 |
|
|
|
1st Experiment. Investigate oxidizing properties of Potassium Permanganate (KMnO4) depending from pH
Materials and Reagents : water, solutions of 2N sulfuric acid (H2SO4), 0,1N sodium hydroxide (NaOH), 2N potassium permanganate (KMnO4) and sodium sulfite (Na2SO3), test tubes and disposable plastic beral pipets
Potassium permanganate consists of dark purple or bronze-like crystals and it is a strong oxidizing agent. It is used extensively in laboratory work and also in dyeing wood, bleaching, photography, and tanning. Dilute solutions are mildly irritating to the skin and high concentrations are caustic. Potassium permanganate stains skin and clothing like silver nitrate.
Oxidizing properties of Potassium permanganate is depended from pH:
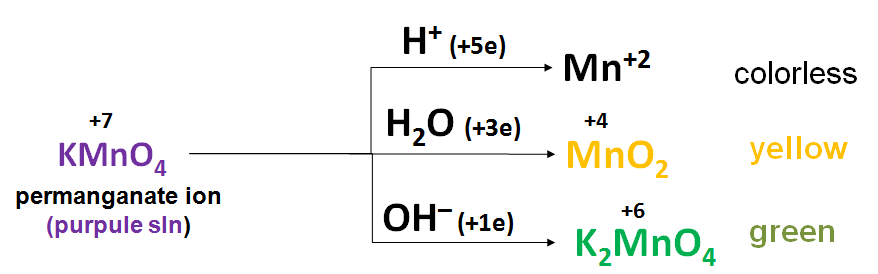
PROCEDURE:
1) Take three test tubes and sign them as 1st, 2nd, 3rd
2) Measure by volumetric tube 2 ml KMnO4 solution and pour into all test tubes, then diluted 1st tube with 5 – 7 drops of sulfuric acid, 2nd tube with 5 – 7 drops of distilled water and 3rd tube with 5 – 7 drops of sodium hydroxide.
3) Measure by volumetric tube 2 ml of sodium sulfite (Na2SO3) and mix into each tubes with permanganate. At the same time, solutions in tubes begin to change their colors. In acidic solution (pH<7) permanganate ion is colorless, in neutral medium (pH=7) solution it is yellow and in basic medium (pH>7) solution it is green.
4) Write a balanced redox reaction between KMnO4 and Na2SO3 in acidic, neutral and basic solutions. Determine the oxidation number for each atom. Identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, and their calculate equivalent masses:
a) KMnO4 + H2SO4 + Na2SO3 ®
b) KMnO4 + H2O + Na2SO3 ®
c) KMnO4 + NaOH + Na2SO3 ®
|
|
|
2nd Experiment. Investigate oxidizing and reducing properties of iron (III, II)compounds
Materials and Reagents : solutions of potassium iodide (KI), iron (III) chloride (FeCl3), 2N sulfuric acid (H2SO4), 2N potassium permanganate (KMnO4), iron (II) sulfate (FeSO4), 1% starch solution, test tubes and disposable plastic pipets.
A) Property of compound iron (Fe3+)
1) Take two test tubes.
2) In the 1st test tube measure by volumetric tube 2 ml solution of iron (III) chloride (FeCl3).
3) In the 2nd test tube measure by volumetric tube 2 ml solution of potassium iodide (KI).
4) In both test tubes add 2 drops of 1% starch solution (solutions in tubes does not change).
5) Then mix both solutions in tubes: FeCl3 + KI. Solution colouring in dark blue. Why? What is a product of reaction and colour of it?
6) Write a balanced redox reaction between KI and FeCl3 [among the products are I2 and Fe2+]. Determine the oxidation number for each atom. Identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, and their calculate equivalent masses:
FeCl3 + KI ®
B) Property of compound iron (Fe2+)
1) In test tube measure by volumetric tube 2 ml KMnO4 solution, then add 5-6 drops of H2SO4 and solution of FeSO4 until solution is colourless.
2) Write a balanced redox reaction between KMnO4 and FeSO4 in acidic solution [among the products are Mn2+ and Fe3+]. Determine the oxidation number for each atom. Identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, and their calculate equivalent masses:
KMnO4 + H2SO4 + FeSO4 ®
3rd Experiment. Investigate Disproportionation reaction
Materials and Reagents : solutions of 2N sulfuric acid (H2SO4), 0,1N sodium hydroxide (NaOH), iodine water (I2), test tubes and disposable plastic beral pipets
PROCEDURE:
1) In test tube measure by volumetric tube 2 ml light brown iodine water (I2), then add 5-6 drops of sodium hydroxide (NaOH) and mixed solution until it is colourless. What happens in solution?
2) Write a balanced redox reaction between I2 and NaOH. Determine the oxidation number for each atom. Identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, and their calculate equivalent masses:
|
|
|
I2 + NaOH ®
3) Then in that tube add some drops of sulfuric acid (H2SO4) until solution colour in light brown again. Why? What forms in solution and colour of it?
4) Write a balanced redox reaction. Determine the oxidation number for each atom. Identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, and their calculate equivalent masses.
EXERCISES:
1. Determine the oxidation number for each atom in the formulas: Ca3(PO4)2, Al2(SO3)3, (NH4)2S, P, Cl2O7, H3[PtCl6], (Cr2O7)2-, (CO3)2-
2. Write balanced equations for the complete combustion of a) C8H18(l), b) CH3OH(l), and c) C3H7SH(l).
3. Classify each of these reactions with respect to the following categories: combination reaction, decomposition reaction, combustion reaction, and single‑displacement reaction.
a) 2Fe2O3 (s) + 3C (s) ® 4Fe (l) + 3CO2(g)
b) Cl2 (g) + F2 (g) → 2ClF(g)
c) 2Pb(NO3)2 (s) + 4NO2(g) + 2PbO (s) + O2 (g)
d) C5H11SH (l) + 9O2 (g) → 5CO2 (g) + 6H2O (l) + SO2 (g)
4. Electrons are _____________ found unattached to atoms. Thus, for one element or compound to lose electrons and be _____________, another element or compound must be there to gain the electrons and be _____________. In other words, _____________ (loss of electrons) must be accompanied by _____________ (gain of electrons).
5. Reactions in which electrons are _____________, resulting in oxidation and reduction, are called oxidation-reduction reactions.
6. The separate oxidation and reduction equations are called _____________.
7. A(n) _____________ is a substance that loses electrons, making it possible for another substance to gain electrons and be reduced.
8. A(n) _____________ is a substance that gains electrons, making it possible for another substance to lose electrons and be oxidized.
9. Oxidation is defined as the complete or _____________ loss of electrons, reduction as the complete or partial gain of electrons.
10. Just think of oxidation numbers as tools for keeping track of the _____________ in redox reactions.
11. If any element undergoes a(n) _____________ of oxidation number in the course of a reaction, the reaction is a redox reaction. If an element’s oxidation number _____________ in a reaction, that element is oxidized. If an element’s oxidation number _____________ in a reaction, that element is reduced. The reactant containing the element that is oxidized is the _____________ agent. The reactant containing the element that is reduced is the _____________ agent.
12. In combination reactions, _____________ elements or compounds combine to form one compound.
13. In decomposition reactions, _____________ compound is converted into two or more simpler substances.
14. In a combustion reaction, oxidation is very rapid and is accompanied by _____________ and usually _____________.
15. When any substance that contains carbon is combusted (or burned) completely, the carbon forms _____________.
16. When a substance that contains hydrogen is burned completely, the hydrogen forms _____________.
17. When any substance that contains sulfur burns completely, the sulfur forms _____________.
18. In single‑displacement reactions, atoms of one element in a compound are displaced (or replaced) by atoms from a(n) _____________.
19. Determine the oxidation number for each atom, and decide whether the reaction is a redox reaction or not. If it is redox, identify which substance is oxidized, which substance is reduced, the oxidizing agent, and the reducing agent, balanced equation and calculate equivalent masses of oxidant and reductant:
KCl + HNO3 + O2 → KNO3 + Cl2 + H2O
20. Perform test
| № | Question | Variant |
| Which of the following characterizes the hydrogen. | a. Element losing electrons is losing oxygen, element gaining electrons is gaining oxygen. b. Element gaining electrons is oxidation–reduction relationship? oxidized, element losing electrons is reduced. c. Element gaining electrons is losing hydrogen, element losing electrons is gaining d. Element losing electrons is oxidized, element gaining electrons is reduced. | |
| 2 | What kind of process is represented by this reaction? Na → Na+ + e– | a. oxidation b. reduction c. redox d. neutralization |
| 3 | The chemical equation above shows a redox reaction: Mg + O2 = 2MgO Which of these best represents what has occurred? | a. Magnesium is the oxidizing agent and was reduced. b. Oxygen is the reducing agent and was oxidized. c. Magnesium is the reducing agent and was oxidized. d. Oxygen is the oxidizing agent and was oxidized. |
| 4 | Si + 2Cl2 = SiCl4 The redox reaction above can be used to determine the change in oxidation numbers for chlorine and silicon. According to this equation, what changes occurred? | a. Silicon went from +1 to +4, and chlorine went from +2 to -4. b. Silicon went from 0 to -4, and chlorine went from 0 to +1. c. Silicon went from +1 to -4, and chlorine went from +4 to -4. d. Silicon went from 0 to +4, and chlorine went from 0 to -1. |
LABORATORY WORK
COORDINATION COMPOUNDS
Objectives: To prepare a special type of compound called a coordination compound in the laboratory.
One of the important aspects of any chemical research is the preparation of new compounds. This process is called synthesis. In this experiment you will synthesize a special kind of compound called a coordination compound.
Complexes or coordination compounds are molecules that possess a metal center that is bound to ligands (atoms, ions, or molecules that donate electrons to the metal).
 Coordination complexes (a complex ion) is comprised of two important parts: the central atom and its surrounding ligands. The central atom can be any metallic ion (usually a transition metal). The overall charge can be positive, negative, or neutral.
Coordination complexes (a complex ion) is comprised of two important parts: the central atom and its surrounding ligands. The central atom can be any metallic ion (usually a transition metal). The overall charge can be positive, negative, or neutral.
Coordination compounds are complex or contain complex ions, for example:
· Complex Cation: [CO(NH3)6]3+
· Complex Anion: [CoCl4(NH3)2]−
· Neutral Complex: [CoCl3(NH3)3]°
· Coordination Compound: K4[Fe(CN)6]
A ligand can be an anion or a neutral molecule that donates an electron pair to the complex. Ex: NH3, H2O, Cl-. The number of ligands that attach to a metal depends on whether the ligand is monodentate, bidentate, or polydentate.
A coordination compound consists of a central metal ion which is chemically bonded to one or more atoms or groups of atoms by coordinate covalent bonds. The metal ion contains one or more empty orbitals which can receive pair(s) of electrons and the atom or group of atoms bonded to the metal ion (ligands) contain one or more pairs of electrons which can be donated to the metal ion. When a covalent bond (a bond formed by sharing of one or more pairs of electrons) contains a pair of electrons which comes from only one atom in the bond it is called a coordinate covalent bond.
Coordination compounds are usually brightly colored compounds and some of them play important roles in biological processes; e.g., heme which is found in hemoglobin, is a coordination compound of iron and chlorophyll is a coordination complex of magnesium.
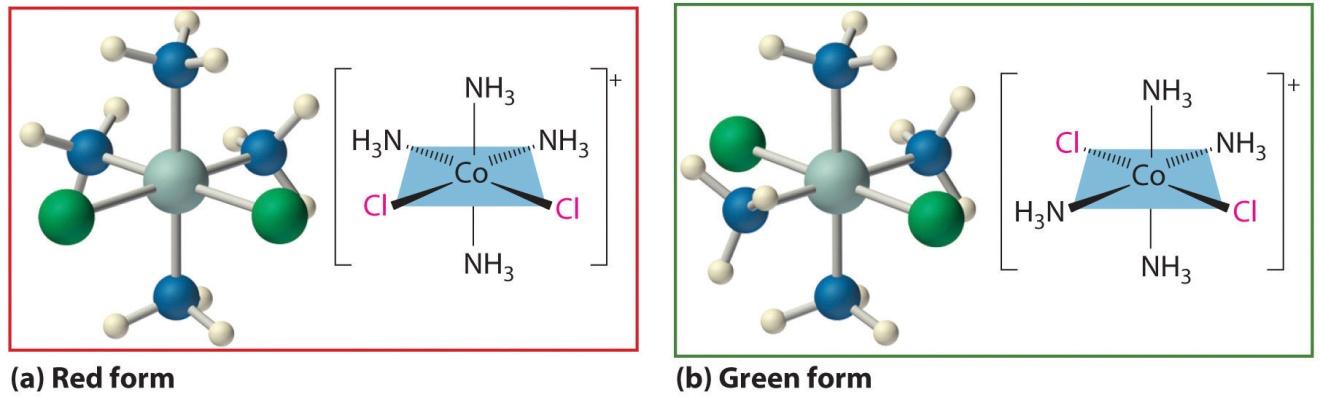
The modern theory of coordination chemistry is based largely on the work of Alfred Werner (1866–1919; Nobel Prize in Chemistry in 1913).
Central Metal Ion. It is an acceptor atom containing vacant orbitals to which a fixed number of ligands are attached via co-ordinate bonds in definite geometrical arrangement.
Coordination Sphere: Central atom and ligands comprise the inner coordination sphere; complex ion enclosed in square bracket, it behaves as a single unit.
Ionization Sphere: Part of compound presents outside coordination sphere, an outer coordination sphere constitutes a positively or negatively charged ions that are on more distance from the central ion or associated there with:
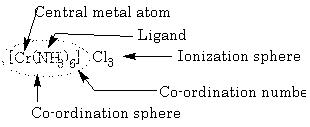
Coordination Number: It is the total number of ligands attached to the central metal atom through coordinate bonds or the number of atoms of a ligand attached to the same central atom, e.g. hexadentate ligand should be counted as forming six co-ordinate bonds.
Oxidation number: It is the charge which the central atom appears to have if all the ligands are removed along with the electron pairs that are shared with the central atom.
[Cr(H2O)4Cl2]NO3 => [Cr(H2O)4Cl2]+ + NO3-
[Cr(H2O)4Cl2]+
x + (4 × 0) + (-1 × 2) = +1 [because the ligand H2O is neutral and 2Cl– carries - 2 charge]
x + 0 - 2 = +1
x = 3 (Cr3+)
Ligands. It is an ion or molecule capable of donating a pair of electrons to the central atom via a donor atom.
Types of ligands:
· Unidentate ligands: Ligands with only one donor atom, e.g. NH3, Cl-, F- etc.
· Bidentate ligands: Ligands with two donor atoms, e.g. ethylenediamine, C2O42-(oxalate ion) etc.
· Tridentate ligands: Ligands which have three donor atoms per ligand, e.g. (dien) diethyl triamine.
· Hexadentate ligands: Ligands which have six donor atoms per ligand, e.g. EDTA.
· Chelating Ligands: Multidentate ligand simultaneously co-ordinating to a metal ion through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation increases the stability of complex. This effect is called chelation effect.
Example:
•• _
H2C — NH2 O = C — O:
| •• | _
H2C — NH2 O = C — O:
The composition and structure of complex compounds:
Дата добавления: 2018-09-20; просмотров: 317; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
