Centrifuge Analytical balance Balance (electronic) Bunsen burner


Crucible tongs Pipets Forceps Funnels




Burner Crucible Graduated Cylinders Buret & Stand Pipets and Bulbs




Volumetric flask Watch dishes Beakers Vials



Petri dish Buret Flasks Desiccator



Drying cabinet Muffle furnace Water bath
WHAT IS CHEMISTRY?
Chemistry is a branch of physical science that studies the composition, structure, properties and change of matter. Chemistry is chiefly concerned with atoms and molecules and their interactions and transformations, for example, the properties of the chemical bonds formed between atoms to create chemical compounds. As such, chemistry studies the involvement of electrons and various forms of energy in photochemical reactions, oxidation-reduction reactions, changes in phases of matter, and separation of mixtures. Preparation and properties of complex substances, such as alloys, polymers, biological molecules, and pharmaceutical agents are considered in specialized fields of chemistry.
Chemistry is the study of matter and the changes that take place with that matter. Matter is everything that you can touch, see, feel, or smell. Matter is defined as anything that has rest mass and volume (space) and is made up of particles. Everything on Earth, everything in our solar system, everything in our galaxy is made up of matter. Because understanding chemistry helps in understand the world around you. The importance of chemistry is that it explains the world around you. If you take classes in chemistry, you'll apply math and logic, which can make studying chemistry a challenge.
 Civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include fermenting beer and wine, extracting metals from ores, extracting chemicals from plants, making alloys like bronze, making glass, making pottery and glazes and for medicines and perfumes. Chemists later began looking for substitutes of natural resources which were essential but rare to obtain and thus began the search for perfect or precise form of experiments. Chemistry is often called the central science because of its role in connecting the physical sciences, biological, medical and environmental sciences.
Civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include fermenting beer and wine, extracting metals from ores, extracting chemicals from plants, making alloys like bronze, making glass, making pottery and glazes and for medicines and perfumes. Chemists later began looking for substitutes of natural resources which were essential but rare to obtain and thus began the search for perfect or precise form of experiments. Chemistry is often called the central science because of its role in connecting the physical sciences, biological, medical and environmental sciences.
|
|
|
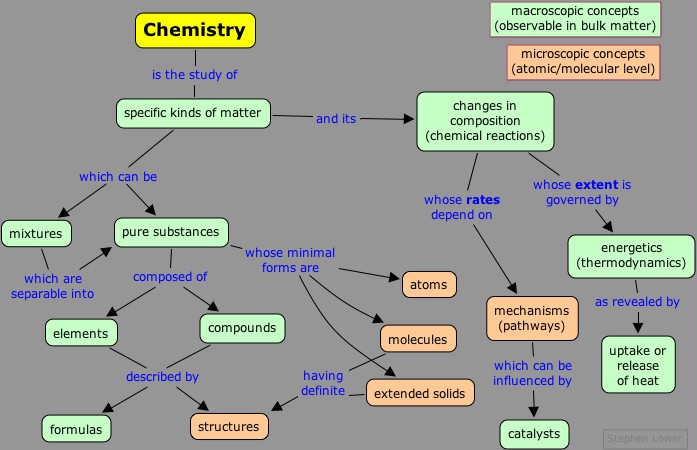
At the most fundamental level, chemistry can be organized along the lines shown here:
· Dynamics refers to the details of that rearrangements of atoms that occur during chemical change, and that strongly affect the rate at which change occurs.
· Energetics refers to the thermodynamics of chemical change, relating to the uptake or release of heat. More importantly, this aspect of chemistry controls the direction in which change occurs, and the mixture of substances that results.
· Composition and structure define the substances that are results of chemical change. Structure refers specifically to the relative arrangements of the atoms in space. The extent to which a given structure can persist itself is determined by energetics and dynamics.
· Synthesis, strictly speaking, refers to formation of new (and usually more complex) substances from simpler ones, but in the present context we use it in the more general sense to denote the operations required to bring about chemical change and to isolate the desired products.
Mastering General Chemistry
1) Success in general chemistry requires a blend of ingredients. It requires a clearly presented body of information which requires lucid instructions.
2) The central principle of modern chemistry revolves around the general ideas of chemistry involving the chemical changes precisely arrangement of molecules.
3) The perfect combinations of atoms into molecules, complicated structures which accounts to the different characteristics of materials.
4) Modern chemistry also emerged very strongly in the 18th century, when researchers began using the systematic balance as a tool in their research.
|
|
|
5) During this time chemists learn to balance essentials in measuring mass, which is nothing but the quantity of matter in the material.
Principles of General Chemistry. Chemists understood the central principle and the true nature of chemicals, atoms, molecules and thus began the journey of shaping molecules to order. The basic principle of Chemistry involves molding of chemical order beginning with chemical reactions, types, the general characteristics of chemicals and the basic stoichiometry involved in turning these simple elements into complex, essential large molecules.
General Chemistry Help. The main intention of introduction to General Chemistry is to provide an introduction to the most important principles and applications of chemistry. Its roots lie in descriptive chemistry, which focuses on the physical and chemical characteristics of the elements and their compounds and stresses a practical knowledge of reactivity and applications. Over the years General Chemistry has become more complex with a strong emphasis on the mathematical and theoretical aspects of science.
Chemistry is the study of nature and its behavior with matter. The atom is the basic building block of matter, representing the smallest unit of a chemical element. An atom in turn is composed of subatomic particles called protons, neutrons and electrons. An atom is classified according to the number of protons and neutrons in its nucleus.
General Chemistry Formulas. A chemical equation is the symbolic representation of a chemical reaction. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers. A chemical equation describes what happens in a chemical reaction. Balancing a chemical equation refers to establishing the mathematical relationship between the quantity of reactants and products. Each side of the chemical equation must represent the same quantity of any particular element.
A chemical formula is a way of conveying data about the proportions of atoms that constitute a particular chemical compound, using a single line of chemical element symbols, numbers, and other symbols, such as dashes, brackets, parentheses, plus (+) and minus (-) signs. From the law of conservation of mass, the mass of the reactants in a reaction must be equal to the mass of the products. More specifically chemical equations must be balanced so that there is the same number of atoms of each element in the products as there is the same number of atoms of each element in the products as there is in the reactants.
|
|
|
General Chemistry Topics. More than thousands of years, human beings have fashioned natural materials into useful products. The topics included in General Chemistry are:
· Elements, Compounds, and Mixtures
· Redox Reactions
· Liquids
· Gases
· Equations of State
· Units & Conversions
· Matter
· Chemical Equations
· Chemical Reactions
· Solutions and mixture
General Chemistry Practice Problems. Below you could see problems. Solved Examples.
Question 1: What is the percentage of lead in galena, PbS? Calculate to 0.1%
Solution: The formula weight of PbS is obtained by adding the atomic weights of lead and sulfur, which we obtain from the standard table.
Weight of one lead atom (1 Pb) = 207.2
Weight of one sulfur atom (1 S) = 32.1
Weight of 1 molecule PbS (galena) = 239.3
Hence 239.3 of PbS contains 207.2 of lead. We see that 100.0g of PbS would contain:

Hence the percentage of lead in galena is 86.6%.
Question 2: A propellant for rocket can be made by mixing powdered potassium perchlorate KClO4, and powdered carbon (carbon black), C with a little adhesive to bind the powdered materials together. What weight of carbon should be mixed with 1000g of potassium perchlorate in order that the products of the reaction be KCl and CO2
Solution: Taking the equation for the reaction as
KClO4 + 2C → KCl + 2CO2
we first calculate the formula weight of KClO4:
weight of 1K = 39.1
weight of 1Cl = 35.5
weight of 4O = 4 * 16.0 = 64.0
Weight of KClO4 = 39.1+35.5+64=138.6
The atomic weight of carbon is 12.0; the weight 2C is 24.0. Hence the weight of carbon required is 24.0/138.6 times the weight of potassium perchlorate:
|
|
|

Hence about 173g of carbon is required for 1000g of potassium perchlorate.
SI UNIT
The first measurements were probably based on the human body (the length of the foot, for example). In time, fixed standards developed, but these varied from place to place. Each country or government (and often each trade) adopted its own units. As science became more quantitative in the seventeenth and eighteenth centuries, scientists found that the lack of standard units was a problem. < They began to seek a simple, international system of measurement. In 1791 a study committee of the French Academy of Sciences devised such a system. Called the metric system, it became the official system of measurement for France and was soon used by scientists throughout the world. Most nations have since adopted the metric system or, at least, have set a schedule for changing to it.
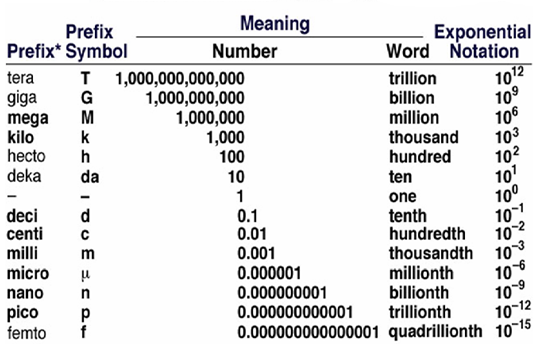
SI Base Units and SI Prefixes. In 1960 the General Conference of Weights and Measures adopted the International System of units (or SI, after the French le Systyme International d’Unites), which is a particular choice of metric units. This system has seven SI base units. In this chapter, we will discuss four base quantities: length, mass, time, and temperature.
One advantage of any metric system is that it is a decimal system. In SI, a larger or smaller unit for a physical quantity is indicated by an SI prefix, which is a prefix used in the International System to indicate a power of 10. For example, the base unit of length in SI is the meter (somewhat longer than a yard), and 10-2 meter is called a centimeter. Thus 2.54 centimeters equals 2.54*10-2 meters. The SI prefixes used in our manual are presented in Table 1.
| Quantity | Name of unit | Abbreviation |
| Mass | Kilogram | Kg |
| Lenght | Meter | m |
| Temperature | kelvin | K |
| Amount of substance | Mole | Mol |
| Time | Second | S |
| Electric current | Ampere | A |
Some quantities are expressed as a function of more than one fundamental units known as derived units. For example velocity, acceleration, work, energy etc. The quantities used in this manual are presented in Table 2.
| Quantity with Symbol | Unit (S.I.) | Symbol |
| Velocity (v) | Metre per sec | ms-1 |
| Area (A) | Square metre | m2 |
| Volume (V) | Cubic metre | m3 |
| Density (r) | Kilogram m-3 | Kg m-3 |
| Energy (E) | Joule (J) | Kg m2s-2 |
| Frequency (n) | Hertz | Cycle per sec |
| Pressure (P) | Pascal (Pa) | Nm-2 |
| Electrical charge | Coulomb (C) | A-s (ampere – second) |
Length, Mass, and Time
The meter (m) is the SI base unit of length. By combining it with one of the SI prefixes, you can get a unit of appropriate size for any length measurement. For the very small lengths used in chemistry, the nanometer (nm; 1 nanometer =10-9 m) or the picometer (pm; 1 picometer = 10-12 m) is an acceptable SI unit. A non-SI unit of length traditionally used by chemists is the angstrom ( Е ), which equals 10-10 m. (An oxygen atom, one of the minute particles of which the substance oxygen is composed, has a diameter of about 1.3 A. If you could place oxygen atoms adjacent to one another, you could line up over 75 million of them in 1 cm.)
The kilogram (kg) is the SI base unit of mass, equal to about 2.2 pounds. This is an unusual base unit in that it contains a prefix. In forming other SI mass units, prefixes are added to the word gram (g) to give units such as the milligram (mg; 1 mg = 10-3 g).
The second (s) is the SI base unit of time. Combining this unit with prefixes such as milli-, micro-, nano-, and pico-, you create units appropriate for measuring very rapid events. The time required for the fastest chemical processes is about a picosecond, which is on the order of how fast supercomputers can perform a single calculation.
When you measure times much longer than a few hundred seconds, you revert to minutes and hours, an obvious exception to the prefix–base format of the International System.
Temperature is difficult to define precisely, but we all have an intuitive idea of what we mean by it. It is a measure of “hotness.” A hot object placed next to a cold one becomes cooler, while the cold object becomes hotter. Heat energy passes from a hot object to a cold one, and the quantity of heat passed between the objects depends on the difference in temperature between the two. Therefore, temperature and heat are different, but related, concepts.
A thermometer is a device for measuring temperature. The common type consists of a glass capillary containing a column of liquid whose length varies with temperature. A scale alongside the capillary gives a measure of the temperature. The Celsius scale (formerly the centigrade scale) is the temperature scale in general scientific use. On this scale, the freezing point of water is 0°C and the boiling point of water at normal barometric pressure is 100°C. However, the SI base unit of temperature is the kelvin (K), a unit on an absolute temperature scale. (See the first margin note on the next page.) On any absolute scale, the lowest temperature that can be attained theoretically is zero. The Celsius and the Kelvin scales have equal-size units (that is, a change of 1°C is equivalent to a change of 1 K), where 0°C is a temperature equivalent to 273.15 K. Thus, it is easy to convert from one scale to the other, using the formula: 
where TK is the temperature in kelvin and tC is the temperature in degrees Celsius. A temperature of 20°C (about room temperature) equals 293 K.
Volume. Volume is defined as length cubed and has the SI unit of cubic meter (m3). This unit is too large a unit for normal laboratory work, so we use either cubic decimeters (dm3) or cubic centimeters (cm3, also written cc). Traditionally, chemists have used the liter (L), which is a unit of volume equal to a cubic decimeter (approximately one quart).
In fact, most laboratory glassware is calibrated in liters or milliliters (1000 mL = 1 L). Because 1 dm equals 10 cm, a cubic decimeter, or one liter, equals (10 cm)3 = 1000 cm3. Therefore, a milliliter equals a cubic centimeter. In summary
1 L = 1 dm3 and 1 mL = 1 cm3
Density. The density of an object is its mass per unit volume. You can express this as 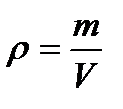
where r is the density, m is the mass, and V is the volume. Suppose an object has a mass of 15.0 g and a volume of 10.0 cm3. Substituting, you find that
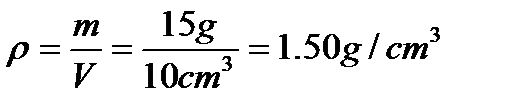
The density of the object is 1.50 g/cm3 (or 1.50 g*cm-3).
Density is an important characteristic property of a material. Water, for example, has a density of 1.000 g/cm3 at 4°C and a density of 0.998 g/cm3 at 20°C. Lead has a density of 11.3 g/cm3 at 20°C. Oxygen gas has a density of 1.33*10-3 g/cm3 at normal pressure and 20°C. (Like other gases under normal conditions, oxygen has a density that is about 1000 times smaller than those of liquids and solids.) Because the density is characteristic of a substance, it can be helpful in identifying it.
Density can also be useful in determining whether a substance is pure. Consider a gold bar whose purity is questioned. The metals likely to be mixed with gold, such as silver or copper, have lower densities than gold. Therefore, an adulterated (impure) gold bar can be expected to be far less dense than pure gold.
Question 2: A colorless liquid, used as a solvent (a liquid that dissolves other substances), is believed to be one of the following:
| Substance | Density (in g/mL) |
| n-butyl alcohol | 0.810 |
| ethylene glycol | 1.114 |
| isopropyl alcohol | 0.785 |
| toluene | 0.866 |
To identify the substance, a chemist determined its density. By pouring a sample of the liquid into a graduated cylinder, she found that the volume was 35.1 mL. She also found that the sample weighed 30.5 g. What was the density of the liquid? What was the substance?
Solution: You substitute 30.5 g for the mass and 35.1 mL for the volume into the equation: 
The density of the liquid equals that of toluene.
Table 3 - Common demical prefixes used with SI Units.
LAB O R A TORY WORK
CHEMICAL THERMODYNAMICS
Objective : to determine the thermal effect of neutralization reaction of strong acid by strong base by calorimetric method
Materials Required: Calorimeter, Thermometer, Stirrer
Reagents: acids – HCl, HNO3, H2SO4, base – NaOH
Thermodynamics is the branch of chemistry which deals with all changes in energy or transfers of energy which accompany chemical or physical changes.
Chemical thermodynamics is the branch of physical chemistry which deals with transport of heat either as a result of chemical change or physical change.
The thermal effect of chemical reaction is the amount of heat which absorbed or released as a result of this reaction.
 The heat of neutralization of an acid is defined as the amount of heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to form water and a salt. Similarly the heat of neutralization of a base is the amount of heat evolved when 1 g equivalent of the base is completely neutralised by a strong acid in a dilute solution:
The heat of neutralization of an acid is defined as the amount of heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to form water and a salt. Similarly the heat of neutralization of a base is the amount of heat evolved when 1 g equivalent of the base is completely neutralised by a strong acid in a dilute solution:
HA + BOH = AB + H2O + 57,3 kJ
For a constant pressure (P=const), calorimeter measures the heat effects of variety of reactions such as neutralisation reactions, heat of solution and heat of dilutions. A coffee cup calorimeter is basically constructed from a polystyrene cup with a lid, in which, the cup is filled with a known amount of water and a thermometer inserted measures the heat changes associated with the reaction.
Heat Q is given the system and consumed to increase the internal energy DU and to perform work against external forces A:
Q = D U + A
For isobar-isothermal process: p, Т = const Аgas = р × D V
D U = U2final — U1initial
D V = V2final – V1 initial
Q = D U + A
Q = (U2 — U2) + ( р V2 — р V1)
Q = (U2 + р V2 ) — (U1 + р V1)
U + pV = H – Enthalpy function
Hess's law (the law of constant heat summation). This law was presented by Hess in 1840. According to this law “If a chemical reaction can be made to take place in a number of ways in one or in several steps, the total enthalpy change (total heat change) is always the same, i.e. the total enthalpy change is independent of intermediate steps involved in the change”. The enthalpy change of a chemical reaction depends on the initial and final stages only.
Q = - D Н
The heat of reaction (or enthalpy of reaction) is actually the difference between the enthalpies of the products and the reactants when the quantities of the reactants indicated by the chemical equation have completely reacted. Mathematically, Enthalpy of reaction (heat of reaction):
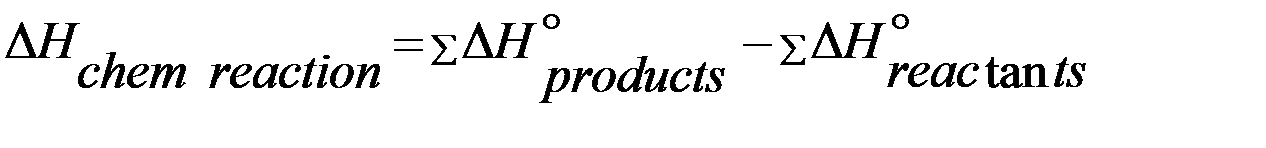

Applications of Hess's law:
a) For the determination of enthalpies of formation of those compounds which cannot be prepared directly from the elements easily using enthalpies of combustion of compounds.
b) For the determination of enthalpies of extremely slow reactions.
c) For the determination of enthalpies of transformation of one allotropic form into another.
d) For the determination of bond energies:
ΔHreaction = S Bond energies of reactants – S Bond energies of products.
e) For the determination of resonance energy.
f) For the determination of lattice energy.
If the reaction is generated heat (Q>0), enthalpy of the system is lowered (DH <0), in this case the reaction is called exothermic.
Examples: (1) C(s) + O2(g) –> CO2(g) + 393.5 kJ (at constant temperature and pressure)
or C(s) + O2(g) –> CO2(g), ΔH = –393.5 kJ
(2) H2(g) + 1/2 O2(g) –> H2O(l), ΔH = –285.8 kJ
(3) Fermentation is also an example of exothermic reaction.
If the reaction is absorbed heat (Q<0), the system increases the enthalpy (DH> 0), and it called the endothermic reaction.
Example: (1) N2(g) + O2(g) –> 2NO(g); H = +180.5 kJ
(2) C(s) + 2S(s) –> CS2(l); H = +92.0 kJ
(3) Preparation of ozone by passing silent electric discharged through oxygen is the example of endothermic reaction.
(4) Evaporation of water is also the example of endothermic reaction.
Factors which influence the heat of reaction: There are a number of factors which affect the magnitude of heat of reaction.
1) Physical state of reactants and products: Heat energy is involved for changing the physical state of a chemical substance. For example in the conversion of water into steam, heat is absorbed and heat is evolved when steam is condensed. Considering the following two reactions:
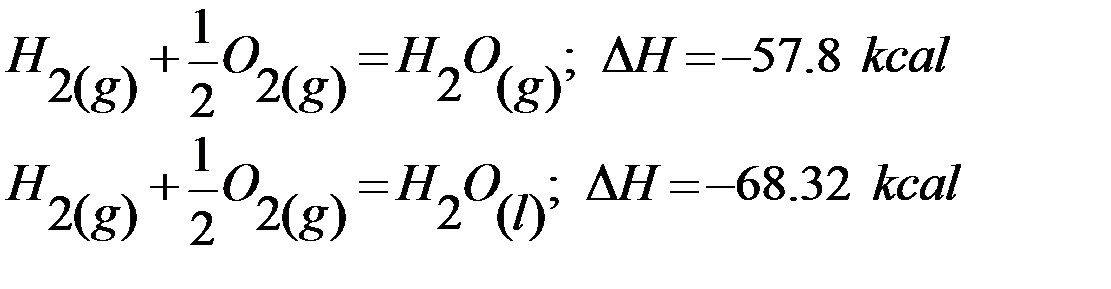
It is observed that there is difference in the value of H if water is obtained in gaseous or liquid state. ΔH value in second case is higher because heat is evolved when steam condenses. Hence, physical sate always affects the heat of reaction.
2) Allotropic forms of the element: Heat energy is also involved when one allotropic form of an element is converted into another. Thus, the value of H depends on the allotropic form used in the reaction. For example, the value of H is different when carbon in the form of diamond or in amorphous form is used.
C (diamond) + O2(g) –> CO2(g), ΔH = –94.3 kcal
C (amorphous) + O2(g) –> CO2(g), ΔH = –97.6 kcal
The difference between the two values is equal to the heat absorbed when 12g of diamond is converted into 12g of amorphous carbon. This is termed as heat of transition.
3) Temperature: Heat of reaction has been found to depend upon the temperature at which reaction is occurring. The variation of the heat of reaction with temperature can be ascertained by using Kirchhoff's equation:
ΔHT2 – HT1/T2 – T1 = CP
Kirchhoff's equation at constant volume may be given as,
ET2 – ET1/T2 – T1 = Cv
Orientation process, or the possibility of chemical reaction is determined by the Gibbs energy. Gibbs energy or the isobaric - isothermal potential – is a manifestation of the cumulative effect of the enthalpy H and entropy S factors:
· At any temperature: 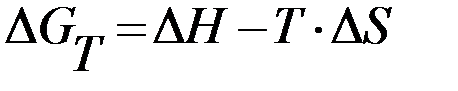
· At standard condition (298K): ΔG = Σ Δ Gproducts ─ Σ Δ Greactants
If ΔG <0, then the reaction is possible.
If ΔG> 0, then the reaction is impossible (is the reverse reaction).
If ΔG = 0, then the system is in equilibrium.
Basic laws of Thermodynamics:
· Zeroth law: If any two systems are in thermal equilibrium with the third system, then they are also in thermal equilibrium with each other.
· First law: First law of thermodynamic states that energy can neither be created nor be destroyed but it can only be converted from one form to another.
· Second law: This law states that “all processes in nature tend to occur with an increase in entropy and the direction of change always lead to the increase in entropy.”
· Third law: This law states that “The entropy of a perfect crystal of each element and a compound is zero at absolute zero.”
PROCEDURE:
The following section gives an example of a laboratory determination of the enthalpy change for chemical reaction in which reactants are mixed in water. After the reaction has taken place the final mixture consists of products, unused reactants and water. The principles of such measurement are as follow.
The reaction is carried out in an insulated container which ideally prevents the reaction mixture from losing heat to (or gaining heat from) the surroundings.
The chemical reaction should be rapid, so that the energy change complete in a short period of time. This is achieved by rapidly mixing the reactants.
An exothermic chemical reaction increases the amount of heat energy contained in the reaction mixture. This raises the temperature of the final mixture by DT°C, defined as Tfinal – Tinitial. An endothermic reaction decreases the amount of heat energy contained in the reaction mixture, so lowering the temperature of the final mixture.
The change in the amount of heat energy due to chemical reaction, Q joules, is calculated using the equation: Q = m*C* D T
(note the negative sign) where m is mass and C (for pure water 4,184*103 J/kg*°C = 1 kal/g*°C) the average specific heat capacity of the final mixture (mainly water) whose temperature is being measured. The value of Q is negative if the chemical reaction is exothermic (when DT is positive); the value of Q is positive if the chemical reaction is endothermic (when DT is negative).
At constant pressure, the heat change undergone produced by the chemical reaction is equal to the enthalpy change of the reaction, i.e. Q = – DH. Note that Q and DH have the different sign.
Task to you : determine the thermal effect of the neutralization reaction, calculate the enthalpy change, please fill out the table and write thermochemical reaction equations:
aA + bB = cC + dD DH>0 (endothermic reaction)
or
aA + bB = cC + dD DH<0 (exothermic reaction)
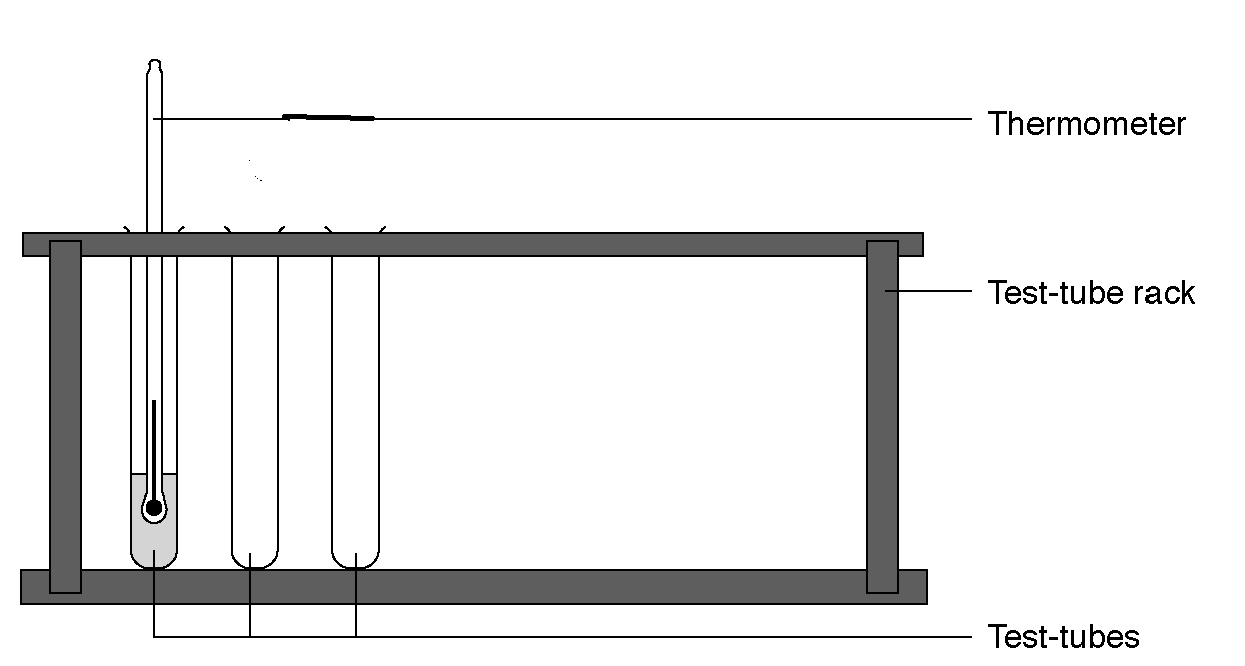
1. Put 5 cm3 of each acid in a test-tube.
2. Record the initial temperature of the each acid in table.
3. Add and mix 5 cm3 of alkali NaOH.
4. Watch what happens? How the temperature of acid is changed: increases or decreases?
5. Carefully stir, using the thermometer, and record the final temperature again.
6. Calculate the thermal effect of neutralization reaction by formula: Q = m*C* D T
7. All measurement results tabulated:
| Volume of acid (5 ml) | Volume of base (5 ml) | Temperature | Dt, °C | Volume of mixture, ml | The heat effects of reaction, kJ/mol | |
| Tfinal | Tinitial | |||||
| HCl | NaOH | |||||
| HNO3 | NaOH | |||||
| H2SO4 | NaOH | |||||
EXERCISE: 1) Use the DH°, S°, DG° data to calculate next quantities of the following reaction:
· heat effect and type of reaction (exothermic or endothermic)
· entropy change under standard conditions (298K)
· determine the possibility of spontaneous reaction under standard conditions (Gibbs free energy)
· determine the possibility of spontaneous reaction at a temperature of 1500 K
· the temperature at which system is being in the equilibrium
2HCl + Mg(OH)2 = MgCl2 + 2H2O
DH°, kJ/mol - 92,30 -924,7 - 644,80 - 285,8
S°, J/mol*K 186,80 63,2 89,5 69,9
DG°, kJ/mol - 95,30 - 833, 8 - 595, 3 - 273,2
2) Perform a test:
| № | Question | Variants |
| 1 | Combustion reactions of fuels is an example of | A) Endothermic reaction B) Exothermic reaction C) Neither Endothermic nor Exothermic D) Both A and B |
| 2 | Exothermic reaction is a chemical reaction that | A) releases heat B) absorbs C) can release heat or absorb heat D) None of the above |
| 3 | Which of the following option states an endothermic reaction | A) Conversion of Graphite to diamond B) Burning of a substance C) Evaporation of water D) Both A and C |
| 4 | Endothermic reaction is a chemical reaction that | A) releases heat B) absorbs C) can release heat or absorb heat D) None of the above |
| 5 | Silver chloride(AgCl) turns grey in sunlight. 2AgCl(s) = 2Ag(s) + Cl2 | A) is an exothermic reaction B) is a decomposition reaction C) is an endothermic reaction D) Both B and C |
| 6 | What form of energy is causing the decomposition reactions | A) heat B) light C) electricity D) All of the above |
LABORATORY WORK
CHEMICAL KINETICS
Objective : to investigate the effect of changing the concentration and temperature on the reaction rate
Materials Required: tubes and graduated tubes, beakers, thermometer, stopwatch, water bath, glass
Reagents: solutions of 0.5%sulfuric acid H2SO4 and 0.5% disodium thiosulfate Na2S2O3, distilled water
Chemical kinetics deals with the study of reaction rate. Every chemical reaction occurs at a definite rate under a given set of conditions. Some reactions are very fast and some other reactions are comparatively slow. Reaction rate can be defined as, "The change in the concentration of a reactant or product per unit time and per unit volume (for homogeneous reactions) or per unit area (for heterogeneous reactions) ":
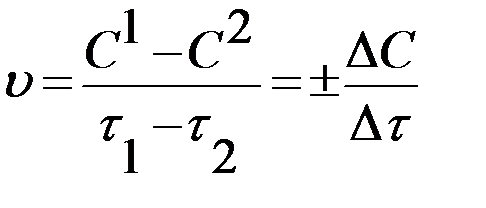
Thus, chemical reaction speed is the reverse quantity of the reaction time. The rate of reactions is measured in terms of change in concentrations of reactants or products mol/l*s. For the reaction to be useful, either in the laboratory or in nature, it must occur at a reasonable rate.
Factors affecting reaction rate:
1) Nature of reactants:
a) Physical state of reactants: This has considerable effect over rate of reaction: Gaseous state > Liquid state > Solid state —> Decreasing rate of reaction
b) Physical size of the reactants: Among the solids, rate increases with decrease in particle size of the solid.
c) Chemical nature of the reactants:
· Reactions involving polar and ionic substances including the proton transfer reactions are usually very fast. On the other hand, the reaction in which bonds is rearranged, or electrons transferred are slow.
· Oxidation-reduction reactions, which involve transfer of electrons, are also slow as compared to the ionic substance.
· Substitution reactions are relatively much slower.
2) Effect of temperature: The rate of chemical reaction generally increases on increasing the temperature. The rate of a reaction becomes almost double or tripled for every rise in temperature.
Temperature coefficient (j) of a reaction is defined as the ratio of rate constants at two temperatures differing by 10°C:
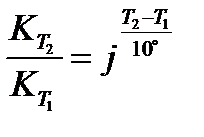
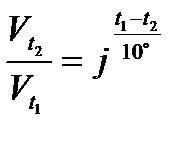 or
or
Arrhenius proposed a quantitative relationship between rate constant and temperature as, 
The equation is called Arrhenius equation. In which constant A is known as frequency factor. This factor is related to number of binary molecular collision per second per litre; Ea is the activation energy; T is the absolute temperature and R is the gas constant. Both A and Ea are collectively known as Arrhenius parameters.
Taking logarithm equation may be written as, log k = log A – Ea/2.303 RT
The value of activation energy (Ea) increases, the value of k decreases and therefore, the reaction rate decreases.
The physical meaning of the activation energy (Ea) is that it is the minimum relative kinetic energy which the reactant molecules must possess for changing into the products molecules during their collision. This means that the fraction of successful collision is equal to 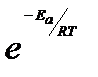 called Boltzmann factor.
called Boltzmann factor.
3) Concentration of reactants (Law of Mass action): The rate at which a substance reacts is directly proportional to its active mass and the rate at which a reaction proceeds is proportional to the product of the active masses of the reacting substances:

• For a reaction, aA + bB –> product
Rate = (dx/dt) ∝ [A]a[B]b; (dx/dt) = k[A]a[B]b
where k is called rate constant or velocity constant.
When [A] = [B] = 1 mol/litre, then dx/dt = k
Thus, rate constant k is also called specific reaction rate.
• The value of rate constant depends on, nature of reactant, temperature and catalyst. It is independent of concentration of the reactants.
• Unit of rate constant = [litre/mol]n–1 × sec–1 = [mol/litre]1–n × sec–1
Where n order of reaction.
4) Presence of catalyst: The function of a catalyst is to lower down the activation energy. The greater the decrease in the activation energy caused by the catalyst, higher will be the reaction rate.
5) Effect of sunlight: There are many chemical reactions whose rate are influenced by radiations particularly by ultraviolet and visible light. Such reactions are called photochemical reactions. For example, Photosynthesis, Photography, Blue printing, Photochemical synthesis of compounds etc.
The radiant energy initiates the chemical reaction by supplying the necessary activation energy required for the reaction.
Types of chemical reactions. On the basis of reaction rates, the chemical reactions have been classified into the following three types,
1) Very fast or instantaneous reactions: These reactions occur at a very fast rate generally these reactions involve ionic species and known as ionic reactions. It is almost impossible to determine the rates of these reactions.
Examples:
a) Precipitation reaction:
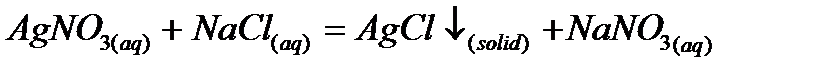
b) Neutralization reaction: 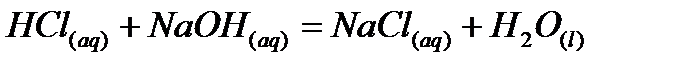
2) Moderate reaction: These reactions proceed with a measurable rates at normal temperature and these reactions are studied in chemical kinetics. Mostly these reactions are molecular in nature.
Examples:
a) Decomposition of: H2O2 : 2H2O2 –> 2H2O + O2
b) Decomposition of: N2O5 : 2N2O5 –> 2N2O4 + O3
3) Very slow reactions: These reactions are extremely slow and take months together to show any measurable change.
Examples:
a) Rusting of iron: 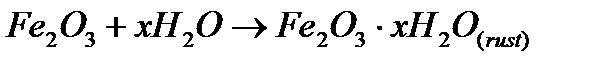
b) 
Chemical Equilibrium. State the irreversible reactions in which the starting materials are fully converted into reaction products, i.e. reaction goes to completion. Signs of the irreversibility:
a) precipitation: Na2SO4 + BaCl2 → 2NaCl + BaSO4↓;
b) the allocation of gas: Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2↑;
c) formation of a weak electrolyte: 2NaOH + H2SO4 → Na2SO4 + H2O.
State the reversible reactions in which the final products interact to form the starting materials.
Such reactions are not going to end, to a state of equilibrium.
Chemical equilibrium – a state of the system in which speed forward and reverse reactions are equal. Equilibrium is called concentration, which are installed on the equilibrium state (for the initial equilibrium concentration of the substance is the amount of material which remained at the time of equilibrium for the reaction products - is the amount of matter, which was formed at the time of equilibrium).
Chemical equilibrium is characterized by the equilibrium constant Кp, which is the ratio of product concentrations of the reaction products to the product of the concentrations of substances in the initial degrees are stoichiometric coefficients. In accordance with the law of mass action for the reversible reaction:
aA + bB → cC + dD
expression of the Кp can be written as follows:
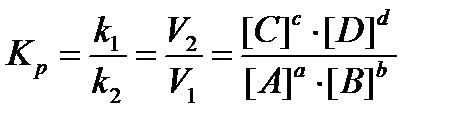
Thermodynamic equilibrium condition: ΔG = 0, ΔF = 0.
Кp shows how many times the rate of direct reaction greater than the rate of reverse reaction:
· If the Кр > 1, the faster the direct reaction; ΔG <0.
· If the Кр <1, then quickly goes back reaction; ΔG> 0.
· If Кр = 1, then ΔG = 0 (equilibrium state).
Кp depends on the nature of the reactants and temperature, and does not depend on the concentration of the catalyst.
Displacement of chemical equilibrium - is the transition system from one equilibrium state to another.
Le Chatelier's principle: if the system is in equilibrium, to produce effects (change the concentration, pressure, temperature), the equilibrium is shifted in the direction of the reaction, which weakens this effect.
Experiment based on the following reaction between sodium thiosulfate and sulfuric acid. As result of reaction after a certain period of time in solution we observe turbidity of the solution, and then the yellow precipitate sulfur:
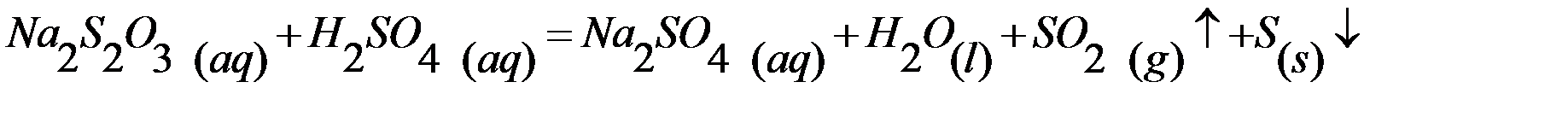
1st Experiment: Investigate the dependence of the rate reaction on the concentration change of reactant.
PROCEDURE:
1) Take four test tubes and sign their as 1st, 2nd, 3rd and 4th, one volumetric tube signed as “Acid”
2) Measure by volumetric tube 2 ml Na2S2O3 solution and pour into “1st“ test tube, then diluted with 6 ml distilled water
3) Measure by volumetric tube signed as “Acid” 2 ml of sulfuric acid H2SO4 and mix into a diluted solution of sodium thiosulfate in “1st“ test tube. At the same time, start the stopwatch for fixing a moment time when appear turbidity of the solution, and then the yellow precipitate sulfur in solution
4) Record the measurements in the table and calculate the reaction rate
5) Repeat experiments with subsequent test tubes
|
| Solution of thiosulfate and water, ml | Sulfuric acid volume, ml | The relative concentration of the solution:
| Time of reaction (turbidity appears) | The relative rate of reaction
| |

| 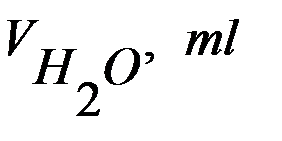
| H2SO4 | ||||
| 1 | 2 | 6 | 2 | |||
| 2 | 4 | 4 | 2 | |||
| 3 | 6 | 2 | 2 | |||
| 4 | 8 | 0 | 2 | |||
Task to you: please, show on the graph the dependence of the rate reaction on the relative concentration of disodium thiosulfate in solution in next coordinates 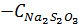 .
.
2nd Experiment: Investigate the dependence of the rate reaction on the temperature change of reactant solution.
PROCEDURE:
1) Take four test tubes and sign them as 1st, 2nd, and 3rd, one volumetric tube signed as “Acid”.
2) Measure by volumetric tube 8 ml Na2S2O3 solution and pour into all test tubes signed as 1st, 2nd and 3rd .
3) Take 1st test tube with thiosulfate solution, insert the thermometer into the test tube, fix the temperature of the solution, and then heat the test tube on a water bath to a temperature of 30°C.
4) At that time, measure by volumetric tube signed as “Acid” 2 ml of sulfuric acid H2SO4 and mix into a heated solution of sodium thiosulfate in “1st“ test tube. At the same time, start the stopwatch for fixing a moment time when appear turbidity of the solution, and then the yellow precipitate sulfur in solution
5) Record the measurements in the table and calculate the reaction rate
6) Repeat experiments with subsequent test tubes.
| № | Na2S2O3 thiosulfate solution volume, ml | H2SO4 sulfuric acid volume, ml | Heating temperature of the solution, t ° C | Time of reaction (turbidity appears)
| The relative rate of reaction

|
| 1 | 8 | 2 | 30°C | ||
| 2 | 8 | 2 | 40°C | ||
| 3 | 8 | 2 | 50°C |
Task to you: please, show on the graph the dependence of the rate reaction on the temperature of solution in next coordinates  .
.
3rd Experiment. Altering an Equilibrium System: Le Chatelier’s Principle
1 KSCN and Fe(NO3)3 form ions in water:
KSCN ↔ K+ + SCN–
Fe(NO3)3 ↔ Fe3+ + 3NO3–
2 Na2HPO4 binds strongly to Fe3+ ions, essentially removing them from solution.
3 KSCN is toxic in high concentrations. Avoid contact with skin.
Purpose:
· To examine the equilibrium: Fe3+ + SCN– ↔ FeSCN2+
(colourless) (brown)
· To use Le Chatelier’s principle to explain changes in the equilibrium
PROCEDURE:
1 Obtain 4 clean test tubes, a clean 50 mL beaker, and a glass stirring rod.
2 Into the beaker place approximately 40 mL of 0.001 M KSCN.
3 Add four drops of Fe(NO3)3 solution to the beaker containing the KSCN. Stir. Rinse off stirring rod.
Colour of KSCN __________, Colour of Fe(NO3)3 ______________,
Colour when the two are added __________. What product/ion provides the colour? ___________
4 Divide the coloured solution into the 4 tubes (the levels only need to be roughly equal).
5 The first test tube will act as a control (i.e. you will compare the colour of the other tubes to tube #1).
6 To tube #2 add some KSCN(s) (about ¼ - ½ a small spoonful). Stir. Record colour change in chart.
7 To tube #3 add three drops of Fe(NO3)3. Stir with clean glass rod. Record any colour change.
8 To tube #4 add 5-10 small crystals of Na2HPO4. Stir with clean glass rod. Record any colour change.
9 Using any of the chemicals in the lab, see if you can return one of the tubes to the colour of tube 1. See if you can change the colour of one of the tubes from brown to colourless and then back to brown.
10 Dump the contents of test tubes and beakers down the sink. Rinse and return all equipment.
| Tube | Addition of | Colour change | Explanation according to Le Chatelier’s principle |
| 2 | KSCN | ||
| 3 | Fe(NO3)3 | ||
| 4 | NaH2PO4 |
11. Which of the tubes has a different Kc value than tube #1? ____________
Explain: _______________________________________________________
12. For each of the tubes indicate how the level of each ion was affected (use ↑ for increased, ↓ for decreased, – for no change). Also, predict which chemicals (KSCN, Fe(NO3)3, and/or Na2HPO4), if any, could be used to return the tubes to exactly their original equilibrium concentrations for all three ions (Fe3+, SCN– and FeSCN2+).
| Tube | Stress imposed in equilibrium | Change from original concentration (↑, ↓, or –) | Chemical that could exactly restore [ ]s | ||
| Fe3+ | SCN– | FeSCN2+ | |||
| 2 | SCN- | ||||
| 3 | |||||
| 4 | |||||
EXERCISE: write down mathematical expressions of the speeds of straight and reverse reaction:
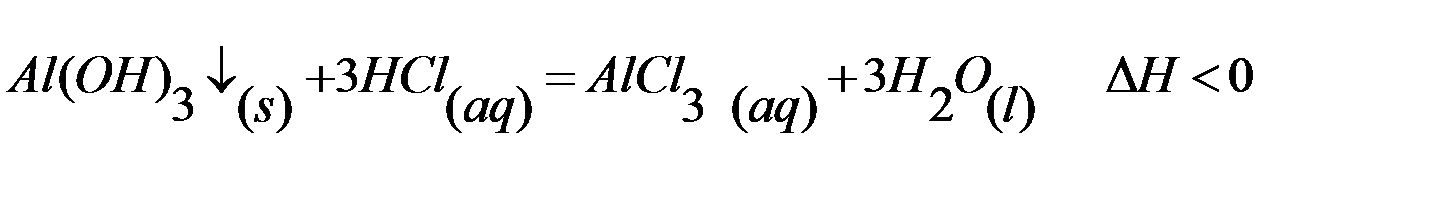
1) Determine the state of the system (homogeneous or heterogeneous)
2) Predict how many times will increase the speeds of straight reaction if you increase the concentration of hydrochloric acid 3 times
3) Write down a mathematical expression for the equilibrium constant for this reaction
4) Predict how to change the direction of the equilibrium with the following changes in the conditions of the reaction: a) if temperature of system increases; b) if pressure decreases; c) if you decrease the concentration of hydrochloric acid; d) if you decrease the concentration of aluminum chloride.
5) Perform test
| № | Question | Variant |
| 1 | Chemical kinetics is the branch of chemistry which deals with the study of: | a) Speed or rate of chemical reaction. b) The factors affecting the rates of the reaction. c) The mechanism by which the reactions proceed. d) All of these. |
| 2 | The unit of the rate of reaction is: | a) mol L-1 min -1 b) mol -1 L min-1 c) Both a and b d) none of these |
| 3 | The rate of reaction depends upon the molar concentration of reactants which: | a) Keep on increasing with passage of time. b) Keep on decreasing with passage of time. c) Remains same with passage of time. d) Does not depend upon the time. |
| 4 | The factors on which the rate of reaction depends is: | a) Temperature. b) Presence of catalyst. c) Presence of light. d) All of these |
| 5 | Activation energy of a chemical reaction can be determined by: | a) Evaluating velocities of reaction at two different temperature. b) Changing concentrations of reactants. c) Evaluating rate constants at two different temperatures. d) Evaluating rate constant at standard temperature. |
| 6 | In the Arrhenius equation K = A exp (-E/RT), A may be termed as the rate constant at: | a) High temperature. b) Infinite temperature. c) Low temperature. d) Moderate temperature. |
| 7 | The rate of a reaction doubles when the temperature changes from 27°C to 37°C. Calculate the energy of activation. | a) 54.6 kJ mol-1 b) 53.6 kJ mol-1 c) 52.6 kJ mol-1 d) 54.1 kJ mol-1 |
| 8 | The role of catalyst in a chemical reaction is to change: | a) Equilibrium constant. b) Activation energy. c) Heat of reaction. d) Products of reaction. |
| 9 | The ionic reactions are very fast because: | a) These reactions are highly exothermic. b) The energy of interaction between charged ions is greater than between neutral molecules. c) It does not involve bond breaking. d) The number of collisions between ions per unit volume per second is very large. |
| 10 | The catalyst used for heterogeneous catalysis is: | a) Solid b) Gas c) Liquid d) None of these |
| 11 | Ionic reactions are: | a) Very slow b) Fast c) Slow d) Very fast |
LABORATORY WORK
SOLUTIONS
Objective : to prepare a solution and determine its concentration
Materials Required: graduated cylinder and beakers, balance, volumetric flasks of 100 ml, 250 ml, glass rod, filter papers, watch glass, densimeters set (areometer)
Reagents: sodium chloride, sodium carbonate, barium chloride, distiller water
A solution is a homogeneous mixture of two (or more) substances, the composition of which may vary between certain limits.
This concept is valid if we take the example of alcohol and water i.e. both substances are in the same phase. But what about sugar syrup, which contains 60% sugar and 40% water? Will the sugar be the solvent? Answer is No, Still the sugar is solute. Then what is the right definition for this case.
Solvent is that component of the binary mixture which is in same physical state as the solution will be. A solution consisting of two components is called binary solution. The component which is present in large quantity is called solvent and the component which is small in quantity is called solute. If both components are in same physical state.
Type of Solutions. All the three states of matter (gas, liquid or solid) may behave either as solvent or solute. Depending on the state of solute or solvent, mainly there may be the following seven types of binary solutions:
| Solute | Solvent | Example |
| Gas | Gas | Air |
| Gas | Liquid | Aerated water (CO2 + H2O) |
| Gas | Solid | Hydrogen in palladium |
| Liquid | Liquid | Alcohol in water, benzene in toluene |
| Liquid | Solid | Mercury in zinc amalgam |
| Liquid | Gas | CO2 dissolved in water |
| Solid | Liquid | Sugar in water, common salt in water |
| Solid | Gas | Smoke |
| Solid | Solid | Various alloys |
For a given solution, the amount of the solute dissolved in a unit volume of solution (or a amount of solvent) is termed as the concentration of the solute. Solutions containing relatively high concentration of solute are called concentrated solutions while those of relatively low concentrations of solute are termed as dilute solutions.
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature. It depends upon the nature of solute and solvent as well as temperature and pressure.

Дата добавления: 2018-09-20; просмотров: 296; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!