Solutions of Gases in Liquids
All gases are soluble in water as well as in other liquids to a greater or lesser extent. The solubility of a gas in liquids depends upon the following factors:
· Nature of the gas
· Nature of the solvent
· Temperature
· Pressure
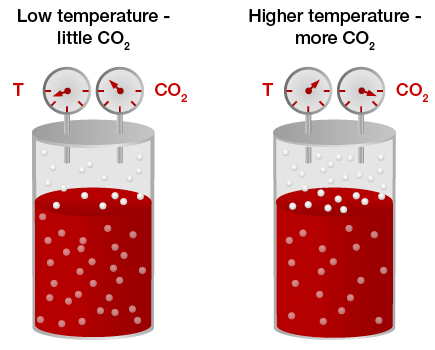 Generally, the gases which can be easily liquefied are more soluble in common solvents. For example, CO2 is more soluble than hydrogen or oxygen in water. the gases which are capable of forming ions to aqueous solutions are much more soluble in water than in other solvents. Gases like hydrogen chloride (HCl) and ammonia (NH3) are highly soluble in water but not in organic solvents in which they do not ionize.
Generally, the gases which can be easily liquefied are more soluble in common solvents. For example, CO2 is more soluble than hydrogen or oxygen in water. the gases which are capable of forming ions to aqueous solutions are much more soluble in water than in other solvents. Gases like hydrogen chloride (HCl) and ammonia (NH3) are highly soluble in water but not in organic solvents in which they do not ionize.
The solubility of most gases in liquids decreases with increase of temperature. When a solution of a gas is heated, the gas is usually expelled. However, some gases are more soluble at higher temperature than at lower.
The most important factor which influences the solubility of a gas in liquid is the pressure. the quantitative connection between the solubility and pressure is given by Henry’s law. According to this law, “The mass of a gas dissolved by a given volume of a liquid, at constant temperature, is proportional to the pressure of the gas”. Or “The solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution”. Or “Mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution”.
The most commonly used form of Henry’s law states that “The partial pressure of the gas in vapour phase (P) is proportional to the mole fraction of the gas (X) in the solution”. Mathematically,
p = KHX
here KH is the Henry’s law constant.
It has been observed that most gases obey Henry ’ s law provided,
· The pressure is not too high.
· The temperature is not too low.
Applications of Henry ’ s law:
· Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. Increased pressure increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous to life. To avoid bends, as well as, the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
|
|
|
· To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
· At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Solutions of Liquids in Liquids
When one liquid dissolves in another, the molecules of the solvent are caused to move apart so as to accommodate the solute molecules. Similarly, the solute molecules must also be separated so that they can take their places in the mixture. In both these processes energy is required. Finally, as the solute and solvent molecules are brought together, energy is released because of the attractive forces between them. When solute and solvent molecules are strongly attracted to each other, more energy is released in the final step. Three cases may arise under these circumstances. The overall dissolution process results either in evolution of heat or absorption of heat, or energy released in the final step is the same as the absorbed in the first two, i.e., net change is zero.
Examples:
· Benzene and carbon tetrachloride : No evolution or absorption of Heat.
· Acetone and water: Evolution of heat.
· Ethyl alcohol and water: Absorption of heat.
A liquid may or may not be soluble in another liquid. Depending upon the relative solubility of a liquid in another, the following three cases are possible.
· Liquids that are completely miscible. Examples: Benzene and toluene; Ethyl alcohol and water; carbon tetrachloride and benzene.
· Liquids that are partially miscible. Examples: Ether and water; Phenol and water; Nicotine and water.
· Liquids that are practically immiscible. Examples: Benzene and water; carbon tetrachloride and water; Benzene and alcohol.
Colloids
A colloid is a dispersion of particles of one substance (the dispersed phase) throughout another substance or solution (the continuous phase). Fog is an example of a colloid: it consists of very small water droplets (dispersed phase) in air (continuous phase). A colloid differs from a true solution in that the dispersed particles are larger than normal molecules, though they are too small to be seen with a microscope. The particles range from about 1*103 pm to about 2*105 pm in size.
|
|
|
Although a colloid appears to be homogeneous because the dispersed particles are quite small, it can be distinguished from a true solution by its ability to scatter light. The scattering of light by colloidal-size particles is known as the
Tyndall effect. For example, the atmosphere appears to be a clear gas, but a ray of sunshine against a dark background shows up many fine dust particles by light scattering. Similarly, when a beam of light is directed through clear gelatin (a colloid, not a true solution), the beam becomes visible by the scattering of light from colloidal gelatin particles. The beam appears as a ray passing through the solution (Figure 2). When the same experiment is performed with a true solution, such as an aqueous solution of sodium chloride, the beam of light is not visible.
Types of Colloids. Colloids are characterized according to the state (solid, liquid, or gas)of the dispersed phase and of the continuous phase. Table 4 listsvarious types of colloids and some examples of each. Fog and smokeare aerosols, which are liquid droplets or solid particles dispersedthroughout a gas. An emulsion consists of liquid droplets dispersedthroughout another liquid (as particles of butterfat are dispersedthrough homogenized milk). A suspension consists of solid particles dispersedin a liquid.
Table 4 -Types of Colloids
| Continuous phase | Dispersed phase | Name | Example |
| Gas | Liquid | Aerosol | Fog, mist |
| Gas | Solid | Aerosol | Smoke |
| Liquid | Gas | Foam | Whipped cream |
| Liquid | Liquid | Emulsion | Mayonnaise (oil dispersed in water) |
| Liquid | Solid | Suspension | AgCl(s) dispersed in H2O |
| Solid | Gas | Solid foam | Pumice, plastic foams |
| Solid | Liquid | Gel | Jelly, opal (mineral with liquid inclusions) |
| Solid | Solid | Solid sol | Ruby glass (glass with dispersed metal) |
|
|
|
FOR PREPARING THE SOLUTION WE NEED:
A volumetric flask is used to make up a solution of fixed volume very accurately. This volumetric flask measures 500 mL ± 0.2 mL. This is a relative uncertainty of 4 x 10-4 or 400 parts per million.
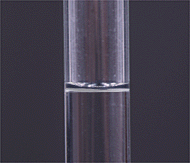
 To make up a solution, first dissolve the solid material completely, in less water than required to fill the flask to the mark.
To make up a solution, first dissolve the solid material completely, in less water than required to fill the flask to the mark.

After the solid is completely dissolved, very carefully fill the flask to the 500 mL mark. Move your eye to the level of the mark on the neck of the flask and line it up so that the circle around the neck looks like a line, not an ellipse. Then add distilled water a drop at a time until the bottom of the meniscus lines up exactly with the mark on the neck of the flask. Take care that no drops of liquid are in the neck of the flask above the mark.

 After the final dilution, remember to mix your solution thoroughly, by inverting the flask and shaking.
After the final dilution, remember to mix your solution thoroughly, by inverting the flask and shaking.
Erlenmeyer flasks and beakers are used for mixing, transporting, and reacting, but not for accurate measurements. The volumes stamped on the sides are approximate and accurate to within about 5%.

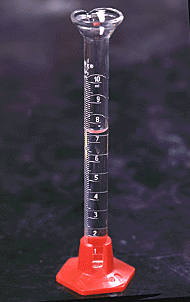 Graduated cylinders are useful for measuring liquid volumes to within about 1%. They are for general purpose use, but not for quantitative analysis. If greater accuracy is needed, use a pipet or volumetric flask.
Graduated cylinders are useful for measuring liquid volumes to within about 1%. They are for general purpose use, but not for quantitative analysis. If greater accuracy is needed, use a pipet or volumetric flask.


Using an Analytical Balance
Turn the balance on by pressing the control bar. The display lights up for several seconds, then resets to 0.0000.

 Place creased, small weighing paper on the balance pan.
Place creased, small weighing paper on the balance pan.
 Close the sliding glass doors. Wait for the green dot on the left to go out. This is the stability indicator light, indicating that the weight is stable.
Close the sliding glass doors. Wait for the green dot on the left to go out. This is the stability indicator light, indicating that the weight is stable.

 Press the control bar to cancel out the weight of the container or paper. The display will again read 0.0000.
Press the control bar to cancel out the weight of the container or paper. The display will again read 0.0000.
Carefully add the substance to be weighed up to the desired mass. Do not attempt to reach a particular mass exactly.
|
|
|

 Before recording the mass, close the glass doors and wait until the stability detector lamp goes out. Record mass of solid.
Before recording the mass, close the glass doors and wait until the stability detector lamp goes out. Record mass of solid.
Solution Definition: A solution is a homogeneous mixture of two or more substances which composed of only one phase. But a solution may exist in any phase.
Aqueous Solution Definition: An aqueous solution is any solution in which water (H2O) is the solvent.
Solvent Definition: The component of a solution that is present in the greatest amount. It is the substance in which the solute is dissolved.
Solute Definition: The substance that is dissolved in a solution. For solutions of fluids, the solvent is present in greater amount than the solute.
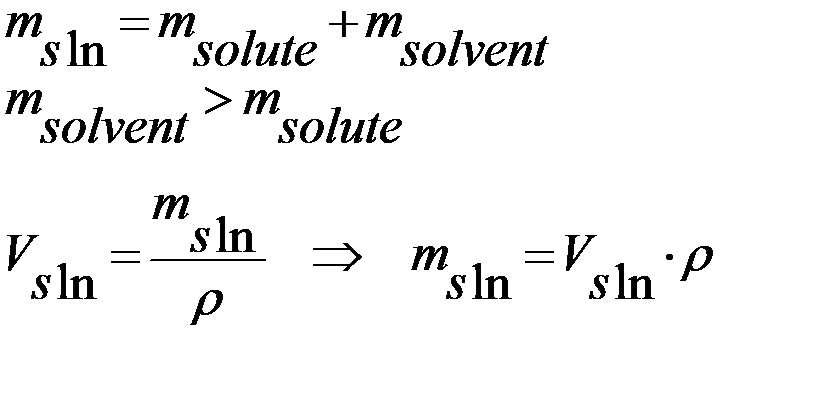
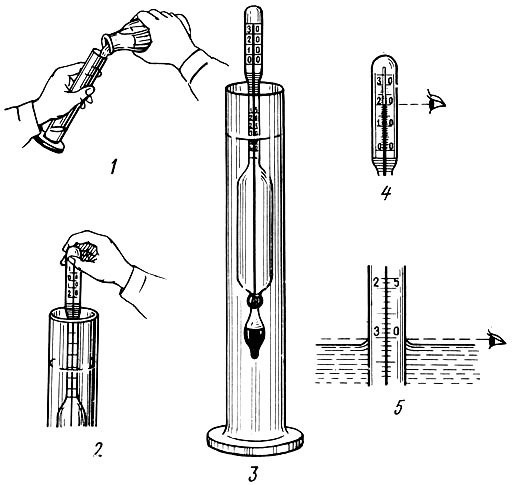
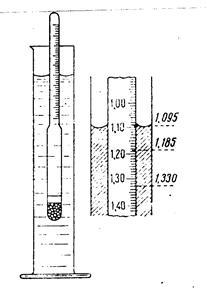
Figure 2 - How to use a densimeter (The density hydrometer measures the density of light or heavy liquid )
PROCEDURE:
1) Weighed in the balance the just about m grams of chemically pure substance (msolute).
2) Fill the volumetric flask of 250 ml about halfway with distilled water or deionized water (Vsln = 250 ml).
3) Transfer the solid salt to the volumetric flask.
4) Rinse the weighing dish with the water to make certain all of the solute is transferred into the flask.
5) Stir the solution until the solute is dissolved. You may need to add more water (solvent) or apply heat to dissolve the solid.
6) Fill the volumetric flask to the mark (meniscus) with distilled or deionized water.
7) Pour the obtained solution into the cylinder to the mark (meniscus) and measure its density by areometer. Write the measurement result (rsln, g/ml)
8) Using the results, calculate the percentage, molarity, normality, molality concentrations and the titer of the solution.
9) Record the measurements into the table:
| № | Solute | msolute, g | Vsln, ml | rsln, g/ml | msln, g | msvt, g | C% | CM, mol/l | CN, mol/l | Cm, mol/kg | T, g/ml |
| 1 | NaCl | 22,67 | 250 | ||||||||
| 2 | Na2CO3 | 10,40 | 250 | ||||||||
| 3 | BaCl2 | 15,80 | 250 |
Scientists use units of concentration to describe the amount of a chemical substance (solute) dissolved in a given volume or mass of liquid. There are many different units for this purpose, including percent by weight or volume, molarity, molality, normality and titer.
When two solutions of the same chemical but having different concentrations are combined, the concentration of the resulting mixture will be different from either of the two starting solutions. You can calculate the concentration of the final mixture using a mathematical formula involving the volumes of the two combined solutions, as well as the initial concentrations of the two solutions.
1) Percent composition by mass is the mass of the solute divided by the mass of the solution, multiplied by 100 (%):
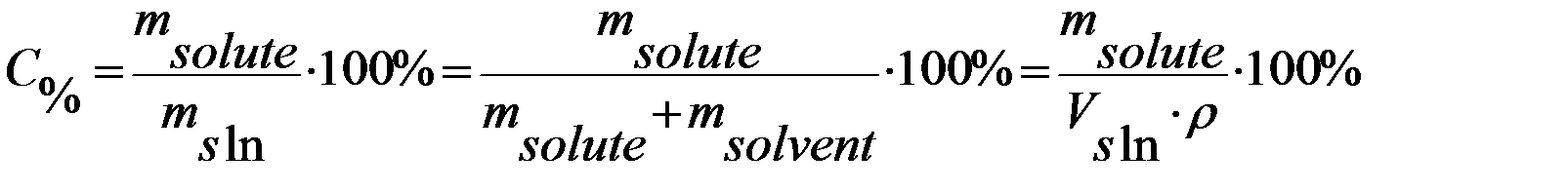
2) Molarity is the number of moles of solute per liter of solution (mol/l):
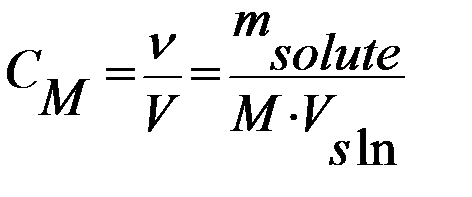
3) Normality is equal to the gram equivalent weight of a solute per 1 liter of solution (mol*eq/l):
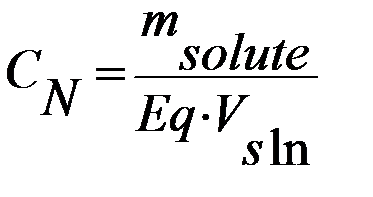
4) Molality is the number of moles of solute per 1 kilogram of solvent (mol/kg):
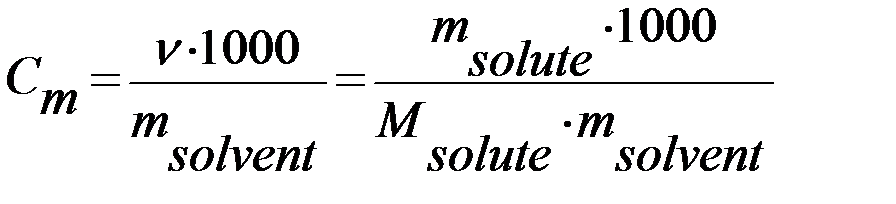
5) Titer is equal to the gram of a solute per 1 milliliter of solution (g/ml):
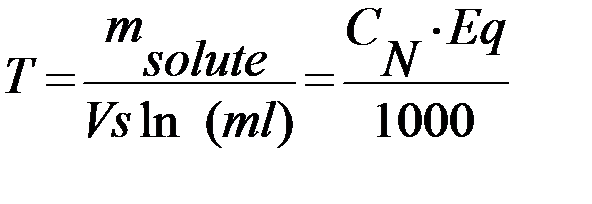
EXERCISES:
1) Calculate the molarity, molality, normality concentrations and titer of 25% aluminum sulfate solution with a density of 1,025g/ml
2) How many grams of salt and water necessary for preparing 650 ml of a 10% solution with a density of 1,005g / ml?
3) How much water should be added to a solution of 40% nitric acid with volume 100 ml and density 1,307g / ml to obtaining a 15% solution of this acid?
4) To neutralize 25 ml of sulfuric acid solution required 0.02N 45.5 ml of sodium hydroxide solution. Calculate the normality and the titer of sulfuric acid solution.
5) An aqueous solution is 8.50% ammonium chloride, NH4Cl, by mass. The density of the solution is 1.024 g/mL. What are the molality, mole fraction, and molarity of NH4Cl in the solution?
6) An aqueous solution is 27.0% lithium chloride, LiCl, by mass. The density of the solution is 1.127 g/mL. What are the molality, mole fraction, and molarity of LiCl in the solution?
7) A sample of potassium aluminum sulfate 12-hydrate, KAl(SO4)2*12H2O, containing 118.6 mg is dissolved in 1.000 L of solution. Calculate the following for the solution:
a. The molarity of KAl(SO4)2.
b. The molarity of SO42-.
c. The molality of KAl(SO4)2, assuming that the density of the solution is 1.00 g/mL.
8) A sample of aluminum sulfate 18-hydrate, Al2(SO4)3*18H2O, containing 159.3 mg is dissolved in 1.000 L of solution. Calculate the following for the solution:
a. The molarity of Al2(SO4)3.
b. The molarity of SO42-.
c. The molality of Al2(SO4)3, assuming that the density of the solution is 1.00 g/mL.
9) A solution is made up by dissolving 15.0 g MgSO4*7H2O in 100.0 g of water. What is the molality of MgSO4 in this solution?
10) A solution is made up by dissolving 15.0 g Na2CO3*10H2O in 100.0 g of water. What is the molality of Na2CO3 in this solution?
11) An aqueous solution is 15.0% by mass of copper (II) sulfate pentahydrate, CuSO4*5H2O. What is the molarity of CuSO4 in this solution at 20°C? The density of this solution at 20°C is 1.167 g/mL.
12) An aqueous solution is 20.0% by mass of sodium thiosulfate pentahydrate, Na2S2O3*5H2O. What is the molarity of Na2S2O3 in this solution at 20_C? The density of this solution at 20°C is 1.174 g/mL.
13) Perform test
| № | Question | Variant |
| 1 | Which of the following factors does not affact the solubility of solids in liquid to large extent? | a) Nature of solvent b) Temperature c) Nature of solute d) Pressure |
| 2 | A solution which remains in contact with undissolved solute is termed as | a) ideal solution b) non-ideal solution c) saturated solution d) unsaturated solution |
| 3 | Which of the following liquid pairs is completely miscible in each other? | a) Benzene and water b) Carbon tetrachloride and water c) Benzene and alcohol d) Ethyl alcohol and water |
| 4 | To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under … | a) high pressure and low temperature b) high pressure and high temperature c) low pressure and high temperature d) low pressure and low temperature |
| 5 | Molarity, normality and molality of a solution is expressed as: | a) the number of moles of a solute present in one litre of the solution. b) the number of moles of a solute present in 1000 gm of the solvent. c) the number of gram equivalent of solute present in one litre of solution. d) the ratio of the number of moles of solute to the total number of moles of solute. |
| 6 | Which of the following is not a volatile substance? | a) Camphor b) Petrol c) Acetone d) Acetanilide |
| 7 | If 50 ml of 0.50 M NaCl solution is diluted with water to a volume of 500 ml the new concentration of solution is: | a) 0.16 M b) 0.05 M c) 0.08 M d) 0.04 M |
| 8 | The value of 0.03 M Ca(OH)2 required to neutralise 20 ml of 0.025 M H3PO4 is | a) 25 ml. b) 50 ml. c) 40 ml. d) 55 ml. |
| 9 | The molarity of 4.6 N H2SO4 solution is: | a) 0.46 M. b) 0.23 M. c) 4.6 M. d) 2.3 M. |
| 10 | Which of the following pair of liquids are immiscible? | a) Acetone + water. b) Benzene + water. c) Ethanol + water. d) Acetic acid + water. |
| 11 | Find the molarity of 0.585g NaCl present in 500 ml of solution. | a) 0.2 M. b) 0.01 M. c) 0.1 M. d) 0.02 M. |
| 12 | Unit of molarity is: | a) Kg / litre. b) mol / litre. c) gm / litre. d) none of these. |
LABORATORY WORK
Дата добавления: 2018-09-20; просмотров: 529; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
