LABORATORY EQUIPMENT AND INSTRUMENTS
Glass is the preferred material for most laboratory purposes. It is resistant to nearly all chemicals except hydrofluoric acid. However, prolonged exposure to strong bases should also be avoided. Only the fluorocarbons are more chemically resistant. Most laboratory glassware is composed of borosilicate glass such as pyrex or kimax. This material has excellent chemical resistance, and may be heated to high temperatures without risk of breaking or significant deformation. Unfortunately, many reagent bottles are made of high sodium glass. These vessels may shatter under extremes of temperature.
Many types of glassware are equipped with ground glass joints. These allow one to assemble all-glass apparatus without the need for corks or rubber stoppers. Bottles made of high sodium glass are often fitted with low-quality ground glass stoppers. These may not seal well, and they cannot be used with the standard interchangeable joints. Borosilicate glassware will have more carefully ground surfaces. The most common joints are the conical type (cone and socket), although spherical joints may also be used. Most conical joints have the "standard taper" of 1 mm change in diameter for every 10 mm in length. Standard taper joints are designated by a two number system. For example, the common "24/40" joint has a maximum diameter of 24 mm and a ground surface length of 40 mm. These are made to be interchangeable, so that any 24/40 cone should fit any 24/40 socket.
Non-volumetric Glassware. The least expensive types of glassware are the non-volumetric pieces. For this reason, operations in the laboratory not requiring precise measurement of volume or those occurring after or prior to volume measurement are best conducted with non-volumetric glassware. Beakers and flasks may be stored either in drawers or cabinets. They are best left upside down so that their interiors are not contaminated by settling dust.
Beakers. Beakers are most often used for mixing solutions, and dissolving solids in water. Solutions in beakers may be protected from atmospheric dust by placing an appropriately sized watch glass over the top.
Erlenmeyer Flasks. Erlenmeyer Flasks are frequently used as the receiving vessel during titrations. Some are made with ground glass stoppered tops. These may be used with reflux condensers (COD test) and other specialized glassware for a variety of purposes.
Reagent Bottles. Reagent bottles may have either flat-topped or round-topped stoppers. The flat-topped stoppers may be placed upside-down on the lab bench while the reagent is being dispensed. The round-topped stoppers must be either held between the fingers or set down on a clean watch glass, or in a clean beaker during use.
|
|
|
Volumetric Glassware. Volumetric glassware always contains one or more circular etched lines. When the bottom of the liquid meniscus just touches that line, the volumetric glassware is ready to either deliver the rated volume (pipets); actually contains the rated volume (flasks); or can be read to determine the precise volume delivered (burets). Volumetric glassware should be read with your eyes at the level of the meniscus, in order to avoid parallax errors. This is especially important when making buret readings.
Volumetric glassware is rated at 20°C and should always be used at or near that temperature. This is necessary, because glass expands and contracts much less than water, so that changes in temperature change the mass of water contained or delivered by volumetric glassware. Burets and pipets should be stored in drawers. Frequently used pipets may be kept upside down on the bench top in a specially designed pipet rack. Volumetric flasks should be stoppered and stored upright in wall cabinets. Volumetric glassware should be dried at room temperature. However, it need not be perfectly dry before re-use. It is a good practice to pre-rinse volumetric glassware in the liquid to be transferred, especially when the glassware is not perfectly dry. There is no need to fill the glassware with liquid, just add a small amount, swirl to wet all surfaces, and discard the rinse solution.
| Technique for reading a meniscus | |
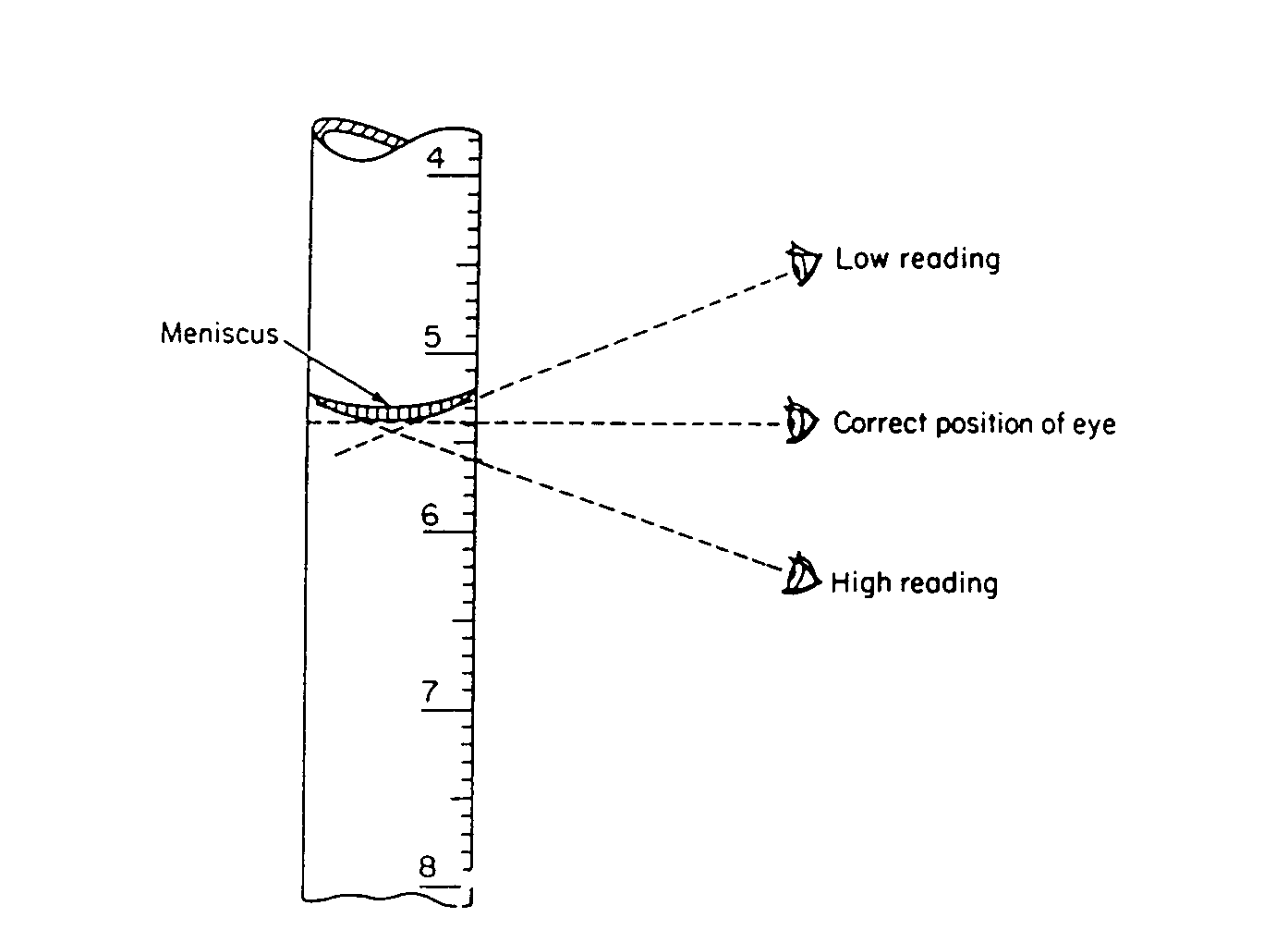 Figure 1 - Technique for reading a meniscus
Figure 1 - Technique for reading a meniscus
| Measure the meniscus at eye level from the center of the meniscus (figure 1). In the case of water and most liquids, the meniscus is concave. Mercury produces a convex meniscus.
|
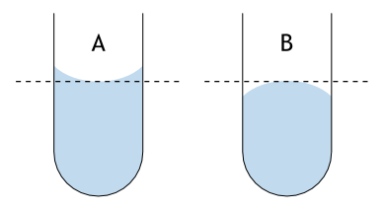
How to read meniscus. The meniscus is the curve seen at the top of a liquid in response to its container. The meniscus can be either concave or convex. A concave meniscus (e.g., water in glass) occurs when the molecules of the liquid are more strongly attracted to the container than to each other. A convex meniscus (e.g., mercury in glass) is produced when the molecules of the liquid are more strongly attracted to each other than to the container.
|
|
|
In some cases, the meniscus appears flat (e.g., water in some plastics). When you read a scale on the side of a container with a meniscus, such as a graduated cylinder or volumetric flask, it's important that the measurement accounts for the meniscus. Measure so that the line you are reading is even with the center of the meniscus. For water and most liquids, this is the bottom of the meniscus. For mercury, take the measurement from the top of the meniscus. In either case, you are measuring based on the center of the meniscus.
Volumetric Pipets. Pipets are used to accurately measure out volumes from 1 mL to 100 mL. Larger volumes can be handled with volumetric flasks; smaller volumes with micropipets and syringes. Two types are available; the highly accurate fixed-volume transfer pipets, and the more flexible measuring pipet. They are filled by creating a suction with a pipet bulb. Never pipet by mouth! We often use Fisher 3-way pipet bulbs. Be careful not to draw liquid into the pipet bulb. If this happens, the $18 bulb may have to be discarded.
Pipets of 1 mL or greater are generally designated "to deliver" (i.e., TD). This means that they will deliver the stated volume when filled to the mark and allowed to drain by gravity while the tip is just submerged or contacting the side of a receiving vessel. You should wait about 20 seconds after all of the liquid has discharged to be sure that the meniscus has reached the correct, equilibrium level. Thus, the last small amount that remains in the tip due to capillary forces must not be forced out. Gently, wipe the outside of the pipet tip with a kimwipe. Note that you should also contact the pipet tip to the vessel while drawing up liquid. Always rinse pipets immediately after use. It is very difficult to remove dryed deposits from the insides of pipets.
Volumetric Flasks. Volumetric flasks are most commonly used for the preparation of standard solutions. Volumetric flasks are normally designated "to contain" (i.e., TC). This means that in order to deliver the stated volume, the liquid must be quantitatively transferred. Volumetric flasks often have round-topped (pennyhead) stoppers which must be either held between the fingers or set down on a clean watch glass, or in a clean beaker while filling or emptying the flask. Some volumetric flasks have polyethylene snap caps in place of ground glass stoppers.
|
|
|
Burets. A buret is a very precisely formed glass tube with calibrated gradations throughout most of its length. Near the lower extremity it contains a stopcock and drain tip. Note from Table 11.1 that the smaller the buret, the smaller the error. Thus you should always use the smallest buret that will completely deliver the total anticipated titrant volume. Proper use of burets will be discussed in the lab #7 handout (Water hardness, acid/base titration).
Graduated Cylinders. Graduated cylinders are commonly used when highest accuracy is not required, especially when odd volumes must be measured. Most also come with polyethylene safe-gard bumpers to prevent breakage in case of tipping.
Buret. Burets are available both with glass and teflon stopcocks. The traditional glass stopcocks must be lubricated with silicone grease. It is usually held in place with a metal tension clip. The teflon stopcocks do not require grease. They are held in place with a teflon washer and nut. The two types of stopcocks are not interchangeable, as they have different tapers. Burets equiped with glass stopcocks will often become contaminated with silicone grease. The beading of water on the inner surface, especially below the stopcock, is indicative of this type of contamination. Be sure that when the buret is full, the tip below the stopcock does not contain any air bubbles. If an air bubble exists here at the start of a titration, it may slowly fill in and introduce significant error. Burets are often used with a loose fitting cap for the purpose of keeping dust out and vapors in.
Be sure that your eyes are at the same height as the meniscus when you read a buret. In this way you minimize error due to parallax. Parallax causes one to overestimate the liquid height when looking at the meniscus from above and vice versa. Be sure that there is no liquid clinging to the inside walls of the buret above the meniscus.
|
|
|
Filtration Apparatus. Filtration, or the separation of solids from liquid, is a very important step in gravimetric analysis. The most common labware used for filtration is the "Millipore-type" apparatus. This consists of a glass or plastic base which supports the filter disc, and a cylindrical reservoir that sits on top of the base. The entire assembly fits on a filter flask by means of a rubber stopper. Typically, the filter flask is connected in series to another filter flask which is ultimately connected to a vacuum. The second filter flask serves as a safety barrier to minimize the chance that liquid will drawn into the vacuum source.
Most environmental filtrations will use either membrane filters or glass fiber filters. The former have better defined pores, and therefore, they result in sharper and more accurate separations. The membrane filters are most commonly used for microbiological determinations. The glass fiber filters, however, are cleaner and more inert than the membrane filters. They are also quite heat resistant. The glass fiber filters are generally used for thermo-gravimetric analyses and for trace organic analysis.
Glassware Cleaning. For the successful analysis of environmental samples, it is imperative that glassware should be scrupulously clean throughout all "crucial" steps of the analysis. Although an experienced analyst will know which steps are crucial, and where a less rigorous cleaning regimen is appropriate, the beginner will not have this advantage. Thus you are advised to always use one of the following procedures:
· General Use. Soak for 10-15 minutes in warm detergent solution. Avoid soaking for very long periods of time, as this may tend to roughen the glass surface (A variety of commercially available laboratory detergents may be dissolved in tap water for this purpose; e.g., 1% Alconox, 2% RBS-35). Rinse 3 times with tap water, followed by 3 rinses with distilled water. In the UMass Environmental Engineering laboratories, we often use a 10% hydrochloric acid rinse just prior to the final 3 rinses with distilled water. Place clean non-volumetric glassware upside down in a 110oC drying oven. Volumetric glassware may deform and loose their precision upon extended heating, therefore, they must be dryed at room temperature. This is the procedure we will be using for most of the experiments in CE 572. The removal of silicone grease presents a special challenge. It should be mechanically removed with kimwipes and pipe cleaners to the extent possible. Chemical removal is best achieved by a series of acid/base soaking cycles. Sometimes and ultrasound bath can be used to remove stubborn particles that may cling to glass surfaces. These devices should be filled with water when in use.
· Trace Organic Analysis. Rinse first with distilled water. Then soak for 20 min in chromic acid bath. Chromic acid may be prepared from commercially available chromerge or according to directions on the bottle (add slowly to a 9 lb bottle of conc. sulfuric acid), or by simply dissolving 25 g of sodium dichromate in 15 mL of water and slowly adding 500 mL of concentrated sulfuric acid. The eventual appearance of green Cr(III) indicates that the solution is exhausted. This cleaning procedure is commonly used for pesticide analysis by gas chromatography. Chromic acid should never be heated! It should always be used in a hood or in a covered bath to prevent atmospheric contamination. Hot chromic acid, or chromic acid contaminated with chloride may give off toxic, and even carcinogenic, fumes. A less toxic substitute called “Nochromix” is commercially available. Although it may be a less powerful oxidant, this product is preferred over chromic acid for reasons of safety.
· Trace Inorganic Analysis. Rinse first with distilled water. Then soak in re-distilled nitric acid. This procedure is commonly used for heavy metal analysis by atomic absorption spectrophotometry. Some soak glassware in a hot dilute (0.004M, pH 12) solution of EDTA for 20 minutes to remove metal ions.
· Oxidant Analysis. Rinse first with distilled water. Then soak overnight with the appropriate oxidant solution. Use a solution that is slightly more concentrated than the unknowns. In some cases it may be most convenient to leave the glassware in a permanent oxidant soak (e.g., chlorine demand free glassware). Other oxidants may be too transient, and glassware must then be prepared immediately before use (e.g., ozone demand free glassware). This procedure is commonly used for the analysis of residual chlorine.




Дата добавления: 2018-09-20; просмотров: 334; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
