Part One. Preparation of the compound.
1. After zeroing the top-loader balance, place a clean, dry 100 mL beaker on the pan and press the bar to set the balance at 0.00 g again (this is called taring the balance). Using the spatula provided, add small amounts of anhydrous copper (II) sulfate to the beaker until you have exactly 2.00 g (to the nearest 0.01 g). Record the weight of the copper (II) sulfate on your report sheet.
2. Add 10 mL of distilled water to the beaker and stir to dissolve the copper (II) sulfate. Leave the stirring rod in the solution. Note the color of the solution, which is due to the copper (II) ion, on your report sheet.
3. Under the hood, add the required volume (as designated by the instructor) of concentrated ammonia to the beaker and stir until any precipitate is completely dissolved. The first addition of ammonia may cause a light blue precipitate of copper (II) hydroxide to form, but this should dissolve to form the tetraammine-copper (II) ion upon further addition of ammonia. Note any color changes on your report sheet which occur during the addition of ammonia. The color of the solution is due to the presence of tetraamminecopper (II) ion.
4. While stirring the solution in the beaker, add 20 mL of ethanol. Fill a 1000 mL beaker half full of crushed ice, position the beaker containing your compound in the crushed ice, and allow its contents to cool for about 10 minutes. Record your observations during the cooling on your report sheet.

Part Two. Isolation of the Compound by Vacuum Filtration.
5. Set up a vacuum filtration flask as shown in the figure to the right. Place a piece of filter paper into the Buchner funnel so that all the holes are covered. Moisten the paper with water. Connect the vacuum tubing from the filter flask to the vacuum outlet at your desk. Turn the vacuum handle until it is in the completely open position. Press down on the Buchner funnel to set the adapter in tightly and ensure a good vacuum.
6. Pour the cold solution in the beaker and the precipitate into the Buchner funnel. When all the liquid has passed through, turn off the vacuum.
7. Pour 5 mL of ethanol into the beaker and rinse any remaining precipitate into the Buchner funnel. Stir CAREFULLY to avoid tearing the filter paper. Turn on the vacuum again and pull the liquid through. Turn off the vacuum and wash the precipitate by adding 5 mL of acetone to the funnel, stirring carefully. Turn on the vacuum and pull the wash liquid through. Repeat the washing one more time with a second 5 mL portion of acetone, turning off the vacuum between washings. After the second washing, allow the suction to continue for 2-3 minutes to speed the during of the product.
8. Remove the product from the Buchner funnel with your scoopula and place it on a paper towel. Spread the product out on the towel and break up any lumps with a stirring rod. Allow the product to air-dry for 15 minutes. Dispose of the liquid in the filter flask (called the filtrate) in the drain under the hood.
9. Tare the top-loader balance with a clean, dry 100 mL beaker and then scrape the dried product into the beaker. Record the weight of the product on your report sheet.
3rd Experiment. Properties of Potassium hexacianoferrate (II)

Potassium ferrocyanide (hexacianoferrate) is the inorganic compound with formula K4[Fe(CN)6] · 3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.
It is a famous reaction which involves treatment with ferric salts to give Prussian blue. Prussian blue is a dark blue pigment with the idealized formula Fe7(CN)18. To better understand the binding situation in this complex compound the formula can also be written as Fe4[Fe(CN)6]3· x H2O. Another name for the color is Berlin blue or, in painting, Parisian or Paris blue.
It was one of the first synthetic pigments used by humans. It is employed as a very fine colloidal dispersion, as the compound itself is not soluble in water. In medicine, Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by cesium and thallium. In particular it was used to absorb 137Cs+ from those poisoned in the Goiania accident. Prussian Blue is orally administered. The therapy exploits Prussian Blue’s ion exchange properties and high affinity for certain “soft” metal cations. Here is the molecular structure of a colloidal dispersion of Prussian Blue:

Prussian blue is formed as a colored precipitate when two solutions of ionic, inorganic compounds are mixed together. There are several different inorganic compounds, which can be used to produce this pigment. In this experiment, the reaction of ferric chloride (FeCl3) and potassium ferrocyanide (K4(Fe(CN)6) will produce Prussian blue:
4FeCl3 + 3K4[Fe(CN)6] à Fe4[Fe(CN)6]3 + 12KCl
intense blue
Reagents: Iron (III) Chloride (FeCl3) and Potassium Ferrocyanide (K4[Fe(CN)6])
Equipment: Balance, Buchner funnel; Filter flask; Tubing; Filter-vac rubber rings (for suction flasks); Scissors and Filter paper
Safety Notes: FeCl3 is an irritant (used to deodorize sewage!) and is hygroscopic. Please keep the bottletightly capped.
PROCEDURE:
1. Prepare a saturated solution of iron (III) chloride by placing 3.7 g FeCl3 in a small beaker with 5 mL distilled water. Use a graduated cylinder to measure the volume of water you used. Stir to dissolve.
2. Separately, prepare a saturated solution of potassium ferrocyanide by placing 1.39 g K4[Fe(CN)6] in another beaker with 5 mL distilled water. Describe the appearance of these solutions in your LNJ.
3. Make the Prussian Blue by pouring the potassium ferrocyanide solution into the beaker with the ferric chloride solution. Stir with a glass rod. Describe in your notebook EXACTLY what you see happening when the solutions are mixed.
4. Obtain filter paper that fits the Buchner funnel (so it lays flat in the bottom). Set up the aspirator by connecting a piece of tubing from the little side-arm on the filter flask to the similar arm sticking out from the side of the faucet. Put the neck of the Buchner funnel through a Filter-vac rubber ring, then seat it into the top of the filter flask. If your filter flask is small, you may want to clamp it into place.
5. Turn on the faucet to create suction. Then pour your reaction mixture into the funnel. Scrape the entire blue product into the funnel: use a little distilled water to rinse the beaker.
6. Once the liquid has all drained into the flask, gently remove the hose from the filter flask to break the suction. Turn off the water. Put your filter paper on a few paper towels inside your lab drawer, and let your Prussian Blue dry until the next lab period. Describe the appearance of the pigment, both while it is wet and after it dries.
Reflections:
1. Write the reaction in ionic form.
2. Decribe structure of complex Prussian Blue and name it.
3. Find several other purposes for this substance, besides as a colorant in paints.
4th Experiment. Properties of Potassium hexacyanoferrate (III)
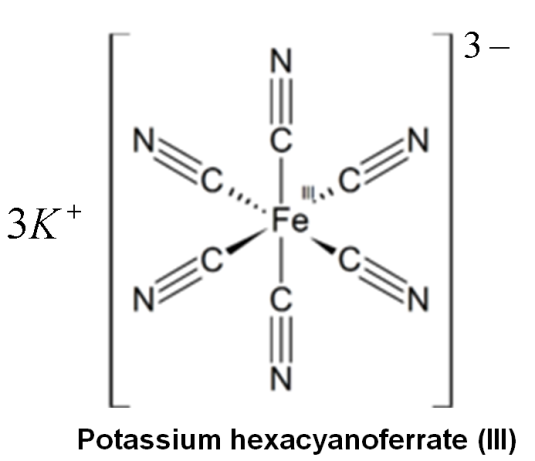 Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence.
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence.
Ferricyanide [Fe(CN)6]3− ion is used to make Turnbull's blue.
In former times, the addition of Fe(II) salts to a solution of ferricyanide was thought to afford a material different from Prussian blue. The product was traditionally named Turnbull's Blue (TB). It has been shown, however, by means of X-ray diffraction and electron diffraction methods, that the structures of PB (Prussian Blue) and TB are identical. The differences in the colors for TB and PB reflect subtle differences in the method of precipitation, which strongly affects particle size and impurity content:
3FeSO4 + 2K3[Fe(CN)6] à Fe3[Fe(CN)6]2 + 3K2SO4
deep blue
Reagents: Iron (II) sulphate (FeSO4) and Potassium Ferricyanide (K3[Fe(CN)6])
Equipment: Balance,Buchner funnel; Filter flask; Tubing; Filter-vac rubber rings (for suction flasks); Scissors and Filter paper
Safety Notes: Potassium ferricyanide; Low toxicity as long as it is not heated, it will release cyanide gas.
PROCEDURE:
1. Weigh accurately about 1.5 g of FeSO4 in a small beaker labeled Turnbull blue and mix with 5 mL of distilled water. Mix the solution until it is all dissolved.
2. Weigh accurately 0.6 g of potassium ferricyanide, K3[Fe(CN)6] into a small beaker and mix with 5 mL of distilled water . Stir until dissolved.
3. Slowly pour the solution of potassium ferrocyanide into the beaker labelled Turnbull Blue and stir the mixture. Let the reaction continue and record the results you see.
4. Weigh accurately a small filter paper and place in the filtration apparatus. Filter the precipitate of TB that forms using the house vacuum.
5. Allow the pigment to dry uncovered on the filter paper until the next lab period in your lab drawer on top of a paper towel.
6. Weigh the dried pigment and filter and subtract the initial weight of the filter paper to obtain the weight of the pigment.
Reflections:
1. Write the reaction in ionic form.
2. Decribe structure of complex Prussian Blue and name it.
3. Find several other purposes for this substance, besides as a colorant in paints.
EXERCISES:
1. Give the systematic names for the following coordination compounds:
a) [Cr(NH3)3(H2O)3]Cl3
b) [Pt(NH3)5Cl]Br3
c) [Pt(H2NCH2CH2NH2)2Cl2]Cl2
d) [Co(H2NCH2CH2NH2)3]2(SO4)3
e) K4[Fe(CN)6]
f) Na2[NiCl4]
g) [Pt(NH3)2Cl4]
h) [Pt(NH3)2Cl2]Cl2
i) Fe(CO)5]
g) (NH4)2[Ni(C2O4)2(H2O)2]
k) [Ag(NH3)2][Ag(CN)2]
l) [CoBr(NH3)5]SO4
m) [Fe(NH3)6][Cr(CN)6]
n) [Co(SO4)(NH3)5]+
o) [Fe(OH)(H2O)5]2+
2. Can you give the molecular formulas of the following coordination compounds?
a) hexaammineiron (III) nitrate
b) ammonium tetrachlorocuprate (II)
c) sodium monochloropentacyanoferrate (III)
d) potassium hexafluorocobaltate (III)
3. Can you give the name of the following coordination compounds?
4. Which of the following is the correct oxidation state of the gold atom in K[Au(CN)2]
a) +2 b) +1 c) -1 d) -2
5. Provide the proper name for the coordination complex [Co(NH3)5Cl]SO4
a) pentaamminechloridocobalt (III) sulfate
b) pentammoniachloridocobalt (III) sulfate
c) sulfoxidepentaamminechloridocobalt (III)
d) pentaamminechloridocobalt (II) sulfate
6. All of the following properties may be affected by cis/trans isomerization of a coordination complex EXCEPT:
a) color of the complex in solution
b) oxidation state of the metal
c) boiling point
d) melting point
7. Which of the following is an example of structural isomerization of a coordination complex?
a) cis to trans isomerization
b) linkage isomerization
c) Λ to Δ isomerization
d) mer to fac isomerization
8. Which of the following is an example of a bidentate ligand?
a) EDTA b) chloride c) ethylenediamine d) carbon monoxide
9. Calculate the coordination number in the complex [Cd(en)2(CN)2]. Note that en = ethylenediamint
a) eight b) six c) four d) two
LABORATORY WORK
ELECTROCHEMICAL CELL TYPES
Learning Objectives:
· Properties and nature of cell will be understood. The differences between electrolytic and electrochemical cells will be learnt. Interconversion of electrical and chemical energy will be emphasized.
· Metal – metal electrode and standard hydrogen electrode construction, the electrode potential and Nernst equation will be learnt.
· Construction of cell with specific electrodes and the EMF calculations will be studied. Daniell cell and it¢s representation. EMF of cell from two half cell potentials will also be calculated.
· Complete electrochemical cell representation and writing oxidation and reduction half cell reactions will be studied with suitable example.
Electrochemistry: the study of the interchange of chemical and electrical energy. Electric charge (q) is measured incoulombs (C). The magnitude of the charge of a single electron (or proton) is 1.602×10-19 C. A mole of electrons therefore has a charge of (1.602×10-19 C)*(6.022×1023/mol) = 9.649×104 C/mol, which is called the Faraday constant, F.

The difference in electric potential between two points measures the work that is needed (or can be done) when electrons move from one point to another:

An electrochemical cell is a system consisting of electrodes that dip into an electrolyte and in which a chemical reaction either uses or generates an electric current, these reactions are called electrochemical, they happen on electrode-solution surface.
Electrode is the material: a metallic rod / bar / strip which conducts electrons into and out of a solution.
An electrode in an electrochemical cell is referred to as either an anode or a cathode:
· Anode: is the electrode where oxidation takes place, it is the negative (-) electrode (example, active metals are soluble in water).
• Cathode: is the electrode where reduction takes place, it is the positive (+) electrode (example, passive metals are insoluble in water).
A half cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally-occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical reaction involves a metal atom in the electrode being dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions.
A standard half cell, used in electrochemistry, consists of a metal electrode in a 1 molar (1 mol/L) aqueous solution of the metal's salt, at 298 kelvin (25 °C). The electrochemical series, which consists of standard electrode potentials and is closely related to the reactivity series, was generated by measuring the between the metal half cell in a circuit with a standard hydrogen half cell, connected by a salt bridge.
1st Experiment. Construction of a half cell (metallic electrode)
If a piece of a metal M is dipped into a solution capable to dissolve it the metal begins to oxidize giving the electrons e- to the metal specimen and forming positive ions M+n transferring to the electrolyte solution. As a result a potential difference (Δφ) between the metal piece and electrolyte forms, it is called electrode potential ([E] = V).
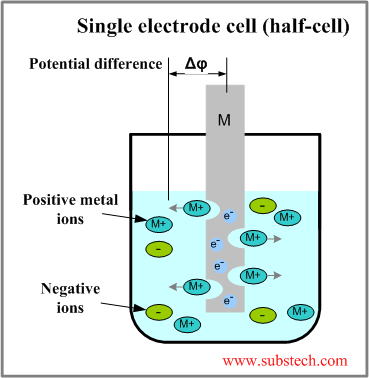 An electrode potential is affected to the nature of electrode, M+n ions concentration in electrolyte solution and temperature. This relationship may be described by Nernst equation (at room temperature 25°C):
An electrode potential is affected to the nature of electrode, M+n ions concentration in electrolyte solution and temperature. This relationship may be described by Nernst equation (at room temperature 25°C):

where E ° is a Standard Electrode Potential. SEP is a measure of the tendency of the metallic electrode to loose or gain electrons when it (metal electrode) is dipped in its own salt solution of unit concentration (1M), at 25°C and atmospheric pressure (1 atm = 101,325kPa).
The absolute value of the potential difference cannot be measured since the measurement would mean inserting another electrode into the electrolyte and formation another potential difference between them. For relative measurements of potentials of various metals in various solutions galvanic cell is used (Figure 2).
Дата добавления: 2018-09-20; просмотров: 301; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
