Preparation of complex compounds
Complex compounds prepared by such reactions:
1) Connection reactions: HgI2 + 2KI → K2[HgI4]
2) Substitution reactions: [Cu(Н2О)4] SO4 + 4NH3 → [Cu(NH3)4] SO4 + 4Н2О
3) Exchange reactions: 2 ZnCl2 + K4 [Fe(CN)6] → Zn2[Fe(CN)6] + 4KCl
4) Redox reactions: 2Al + 6KOH + 6H2O → K3[Al(OH)6] + 3H2↑
Classification of complex compounds
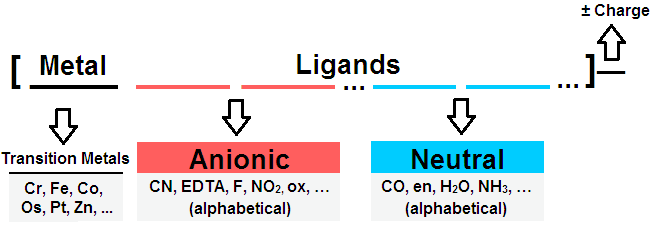
1) By the charge of the complex ion:
| Cationic | Anionic | Neutral |
| [Ag(NH3)2]+Cl | Na[Al(OH)4]- | [Fe(CO)5]° |
| [Cu(NH3)4]2+ SO4 | H2[Pt(Cl)6]2- | [Pt(NH3)2Cl2]° |
| [Co(NH3)4]3+Cl3 | K3[Fe(CN)6]3- | [Cr(H2O)3F3]° |
2) By the nature of the ligand:
| Complex name | Ligand, its name | Example of connection |
| Ammines | NH3 ― ammine | [Zn(NH3)4]SO4 |
| Aquacomplexes | H2O ― aqua | [Al(H2O)6]Cl3 |
| Hydroxocomplexes | OH- – hydroxo | Na2[Zn(OH)4] |
| Carbonilocomplex | CO – carbonil | [Fe(CO)5]° |
| Acidocomplexes | Cl- ― chlorine CN- ― cyanide NO2˗ ― nitrite CO32- ― carbonate SCN- ― thiocyanide SO42- ― sulphate | K3[AlCl6] K4[Fe(CN)6] Na3[Co(NO2)6] [Co(NH3)4CO3] (NH4)2[Hg(SCN)4] K2[Be(SO4)2] |
There are also intracomplex compounds – chelates polynuclear complexes, clathrates, fullerenes. Complex compounds denticity. One characteristic is their denticity ligands. Denticity - is the number of seats occupied by ligands in the inner coordination sphere of the complex.
Complex compounds denticity
| Denticity | Ligands |
| monodentate | NH3, H2O, OH-, Cl-, Br-, F-, NO2-, SCN-, CN- |
| bidentate | CO32-, SO42-, S2O32-, C2O42- |
| polydentate | Aminopolycarboxylic acid (complexones), proteins |
Naming Of Coordination Compounds
Rule 1: To name a co-ordination compound, no matter whether the complex ion is the cation or the anion, always name the cation before the anion.
Rule 2: Part (a) Name the ligand first, in alphabetical order, then the metal atom or ion.
Part (b) For anionic ligands end in “- o -”. For anions that end in “- ide -” (e.g. chloride), “ – ate -” (e.g. sulfate, nitrate) and “ – ite -” (e.g. nitrite), change the ending as follows:-ide —→ -ido, -ate —→ -ato, -ite —→ -ito
Part (c) For neutral ligands, the common name of the molecule is used e.g. H2NCH2CH2NH2 (ethylenediamine). Important exceptions: For H2O —→ aqua, NH3 —→ ammine, CO —→ carbonyl and N2 and O2 are called dinitrogen and dioxygen.
|
|
|
| Anionic ligands | Neutral ligands |
| Br– - Bromo | NH3 – ammine |
| F– - Fluoro | H2O – aqua |
| O2– - Oxo | NO – nitronyl |
| OH– - Hydroxo | CO – Carbonyl |
| CN– - Cyano | O2 – Dioxygen |
| C2O42– - Oxalato | N2 – Dinitrogen |
| CO32– - Carbonato | H2NH2C | H2NH2C — ethylene diamine |
| CH3COO– - aceto |
Name of Metals in Anionic Complexes:
| Iron (Fe2+) | Ferrate | Silver (Ag+) | Agrenate |
| Copper (Cu2+) | Cuprate | Gold (Au3+) | Aurate |
| Lead (Pb2+) | Plambate | Tin (Sn2+) | Stannat |
Rule 3: Greek prefixes are used to designate the number of each type of ligand in the complex ion, di-, tri- and tetra-. If the ligand already contains a Greek prefix (e.g. ethylendiamine) if it is polydenate ligands the prefixes, bis-, tris-, tetrakis-, pentakis- are used instead.
Rule 4: After naming the ligand, name the central metal. If the complex ion is a cation, metal is named same as the element. But if the complex is an anion, the name of metal ends with the suffix –ate- for Latin name.
Rule 5: The oxidation state of the metal in the complex is given as a roman numeral in parentheses.
For example: Name the following coordination complexes
(a) [Cr(NH3)5(H2O)][(NO3)3] (b) [Cr(NH3)4Cl2]Cl
Solution:
(a) The complex contains the [Cr(NH3)5(H2O)]3- ion and three nitrate ions to balance the charge on the complex ion. It is therefore known as pentaammineaquochromium (III) nitrate.
(b) Two of the three chloride ions are ligands that are coordinated to the Cr3+ ion, and the other is a Cl- ion that balances the charge on the [Cr(NH3)4Cl2]+ ion. The compound is therefore known as dichlorotetraamminechromium (III) chloride.
For historic reasons, some coordination compounds are called by their common names. For example, [Fe(CN)6]3- and [Fe(CN)6]4- are named ferricyanide and ferrocyanide respectively, and [Fe(CO)5] is called iron carbonyl.
|
|
|
Writing Formulas of Coordination Complexes
The formula of a coordination complex is written in a different order than its name. The chemical symbol of the metal center is written first. The ligands are written next, with anion ligands coming before neutral ligands. If there is more than one anion or neutral ligand, they are written in alphabetical order according to the first letter in their chemical formula.
In a coordination compound's name, when one of the ions is just an element, the number of atoms is not indicated with a prefix. Since it still has to be written in the formula, it is determined by balancing the overall charge of the compound.
For example: Determine the formulas of the following compounds.
(a) potassium hexacyanoferrate (II)
(b) tris(ethylenediamine)chromium (III) chloride
Solution:
(a) The compound contains enough K+ ions to balance the charge on the negatively charged ferrate ion. Because the ferrate ion is formed from an Fe2+ ion and six CN- ligands, it carries a net charge of -4. The formula for the compound is therefore K4[Fe(CN)6].
(b) The compound contains a Cr3+ ion coordinated to three bidentate ethylenediamine or “en” ligands. It therefore must contain three Cl- ions to balance the charge on the complex ion. Thus, the formula for the compound is [Cr(en)3]Cl3.
Дата добавления: 2018-09-20; просмотров: 480; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
