Using the standard hydrogen electrode
The standard hydrogen electrode is attached to the electrode system you are investigating - for example, a piece of magnesium in a solution containing magnesium ions.
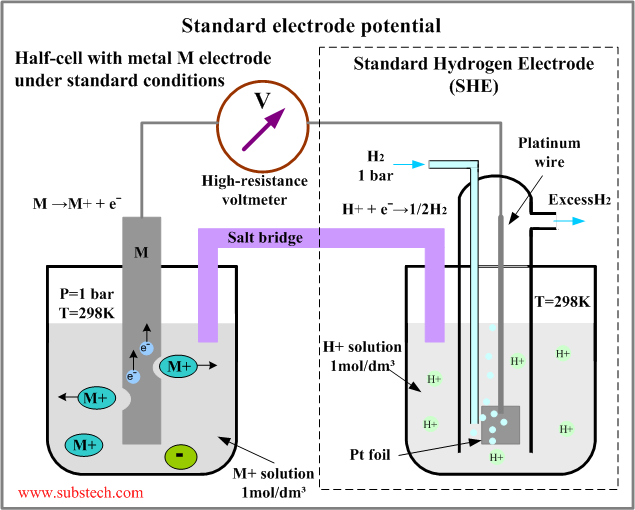 The whole of this set-up is described as a cell. It is a simple system which generates a voltage. Each of the two beakers and their contents are described as half cells.
The whole of this set-up is described as a cell. It is a simple system which generates a voltage. Each of the two beakers and their contents are described as half cells.
The salt bridge is included to complete the electrical circuit but without introducing any more bits of metal into the system. It is just a glass tube filled with an electrolyte like potassium nitrate solution. The ends are "stoppered" by bits of cotton wool. This stops too much mixing of the contents of the salt bridge with the contents of the two beakers.
The electrolyte in the salt bridge is chosen so that it doesn't react with the contents of either beaker.
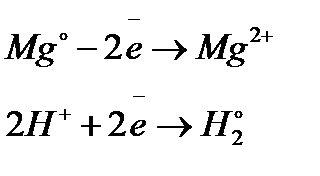 What happens? These two equilibria are set up on the two electrodes (the magnesium and the porous platinum):
What happens? These two equilibria are set up on the two electrodes (the magnesium and the porous platinum):
Magnesium has a much greater tendency to form its ions than hydrogen does. The position of the magnesium equilibrium will be well to the left of that of the hydrogen equilibrium.
That means that there will be a much greater build-up of electrons on the piece of magnesium than on the platinum. Stripping all the rest of the diagram out, apart from the essential bits:
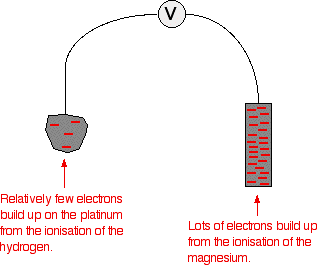 There is a major difference between the charge on the two electrodes - a potential difference which can be measured with a voltmeter. The voltage measured would be DE=2.37 volts and the voltmeter would show the magnesium as the negative electrode (– anode) and the hydrogen electrode as being positive (+ cathode).
There is a major difference between the charge on the two electrodes - a potential difference which can be measured with a voltmeter. The voltage measured would be DE=2.37 volts and the voltmeter would show the magnesium as the negative electrode (– anode) and the hydrogen electrode as being positive (+ cathode).
This sometimes confuses people! Obviously, the platinum in the hydrogen electrode isn't positive in real terms - there is a slight excess of electrons built up on it. But voltmeters don't deal in absolute terms - they simply measure a difference:
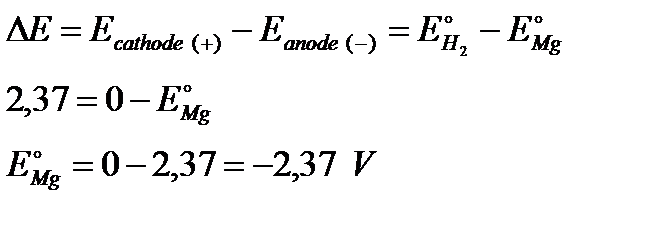
The magnesium has the greater amount of negativeness - the voltmeter records that as negative. The platinum of the hydrogen electrode isn't as negative - it is relatively more positive. The voltmeter records it as positive.
E° values give you a way of comparing the positions of equilibrium when these elements lose electrons to form ions in solution:
· The more negative the E° value, the further the equilibrium lies to the left - the more readily the element loses electrons and forms ions (metal atoms are soluble in own solution and oxidation occurs).
· The more positive (or less negative) the E° value, the further the equilibrium lies to the right - the less readily the element loses electrons and forms ions (metal ions are reduced in solution).
|
|
|
The main type of an electrochemical cell are:
· A voltaic, or galvanic, cell is an electrochemical cell in which a spontaneous reaction generates an electric current.
· An electrolytic cell is an electrochemical cell in which an electric current drives an otherwise non-spontaneous reaction.
2nd Experiment. Construction of Galvanic cell
Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat.
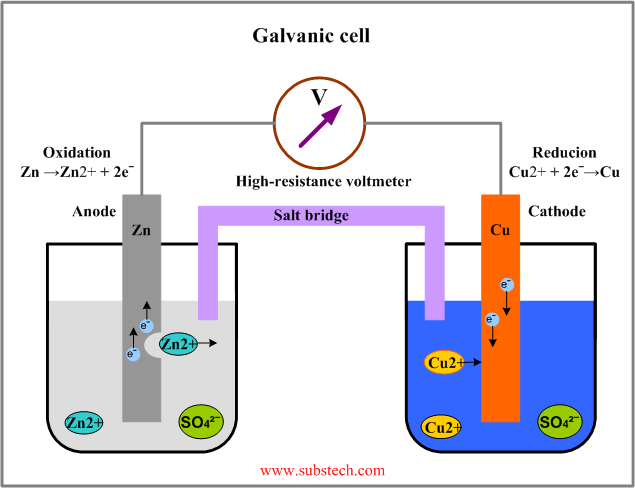
A galvanic Daniell cell consists of two half-cells. Each half-cell has: (1) an electrode, which in the figure are the plates of Zn (zinc) and Cu (copper); and (2) an electrolyte, which in the figure are aqueous solutions of ZnSO4 and CuSO4. The metal of a metallic electrode tends to go into solution, thereby releasing positively charged metal ions into the electrolyte, and retaining negatively charged electrons on the electrode. Thus each half-cell has its own half-reaction. For the Daniell cell, depicted in the figure, the Zn atoms have a greater tendency to go into solution than do the Cu atoms. More precisely, the electrons on the Zn electrode have a higher energy than the electrons on the Cu electrode. Because the electrons have negative charge, to give electrons on it a higher energy the Zn electrode must have a more negative electrical potential than the Cu electrode. However, in the absence of an external connection between the electrodes, no current can flow.
When the electrodes are connected externally (as in the figure, with wire and a light bulb), the electrons tend to flow from the more negative electrode (Zn) to the more positive electrode (Cu). Because the electrons have negative charge, this produces an electric current that is opposite the electron flow. At the same time, an equal ionic current flows through the electrolyte. For every two electrons that flow from the Zn electrode through the external connection to the Cu electrode, on the electrolyte side a Zn atom must go into solution as a Zn2+ ion, at the same time replacing the two electrons that have left the Zn electrode by the external connection.
|
|
|
By definition, the anode is the electrode where oxidation (removal of electrons) takes place, so in this galvanic cell the Zn electrode is the anode. It oxidizes and gives up the electrons of the atoms dissolving in the electrolyte in form of positive ions:
Because the Cu has gained two electrons from the external connection, it must release two electrons at the electrolyte side, where a Cu2+ ion plates onto the Cu electrode. By definition, the cathode is the electrode where reduction (gain of electrons) takes place, so the Cu electrode is the cathode: 
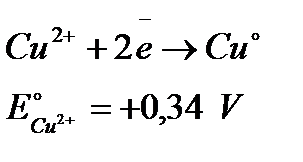
Thus the reaction that is going on is really:
If the value of the electric current is negligible (high resistance voltmeter is used) the measured potential difference between the electrodes is equal to the electromotive force (EMF) of the galvanic cell. The measurement of electromotive force is usually made under standard conditions:
· Temperature: 77ºF (25ºC, 298K);
· Pressure of gases: 1 atm;
· Electrolyte solution activity (concentration): 1 M (1 mol/dm3).
· Electromotive force measured under standard conditions has a notation E°.
EMF of a galvanic cell is a resulting sum of potential differences of the anode and cathode (at standard condition):
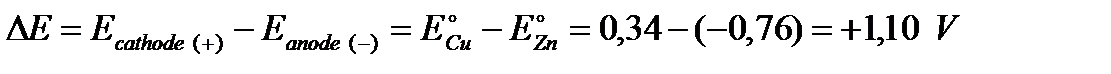
The galvanic cells, as the one shown in the figure, are conventionally described using the following notation:

(anode) (cathode)
where: (s) denotes solid; (aq) means aqueous solution; the vertical bar, /, denotes a phase boundary; and the double vertical bar, //, denotes a liquid junction.
If the cell is operated under non-standard conditions, the potentials must be adapted using the Nernst equation.
3rd Experiment. Construction of concentration cell
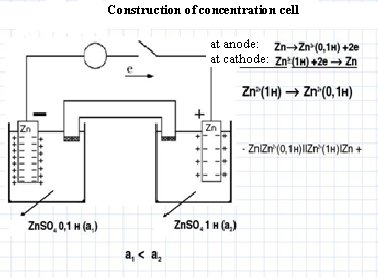
If two plates of the same metal are dipped separately into two solutions of the same electrolyte and are connected with a salt bridge, the whole arrangement is found to act as a galvanic cell.
|
|
|
The cells in which EMF arises due to transfer of matter from one half cells to the other because of a difference in the concentration of species involved are known as concentration cell.
· In these cells, electrodes are identical but these are immersed in solutions of the same electrolyte of different concentrations.
· The source of electrical energy in the cell is the tendency of the electrolyte to diffuse from a solution of higher concentration to that of lower concentration.
· With the expiry of time, the two concentrations tend to become equal. Thus, at the start the EMF of the cell is maximum and it gradually falls to zero. Such a cell is represented in the following manner: (C1 is greater than C2).
· - M / Mn+ (C 2 ) // Mn+ (C 1 ) / M +
· Example: (Zn|Zn2+ (C2))/Anode || (Zn2+ (C1 )|Zn)/Cathode
| (Zn / Zn2+ (C2))/Anode // (Zn2+ (C1) /Zn) / Cathode | |
| Anode (-) | Cathode (+) |
| Zn°(s) – 2e → Zn2+(C2) | Zn2+(C1) + 2e– → Zn°(s) |
· The emf of the cell is given by the following expression at 25o C:
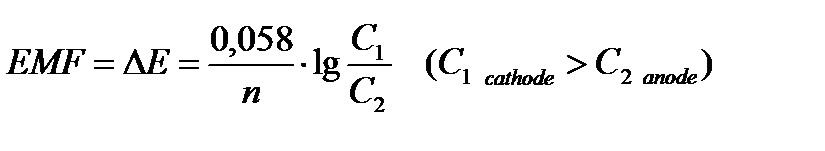
· The concentration cells are used to determine the solubility of sparingly soluble salts, valency of the cation of the electrolyte and transition point of the two allotropic forms of a metal used as electrodes, etc.
· For every concentration cell E° = 0.
EXERCISES:
1) What is the potential of a half cell consisting of aluminum electrode in 0,003N AlCl3 solution 25°C (E°=1,66 V)
2) Determine the standard EMF of the cell: Zn/Zn2+ // Ni2+/Ni
3) Calculate the EMF of the cell: Fe/FeSO4 0,2N // CuCl2 0,001M / Cu
4) Question 1: Which of the following equations represents calomel electrode?
a. Hg2Cl2 + e- 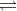 Hg + Cl- b. AgCl (s) + e– → Ag + Cl–
Hg + Cl- b. AgCl (s) + e– → Ag + Cl–
c. Fe3+ + e– → Fe2+ d. H2 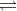 2H+ + 2e-
2H+ + 2e-
Question 2: Magnitude of electrode potential does not depend on.
a. Pressure b. Temperature c. Concentration d. Nature of electrode
|
|
|
Question 3: In which of the following elements is not a component of SHE?
a. Platinum b. Hydrogen c. Mercury d. Gold
Question 4: SHE can be used as..
a. only anode b. only cathode d. both anode and cathode c. neithet anode nor cathode
Question 5: E° value for Cu2+/Cu volt is
a. 0.34 V b. 0.58 V c. 1.02 V d. 1.51 V
Question 6 : Anode of Daniell cell is made up of..
a. Zn b. Cu c. Mg d. Al
Question 7 : At anode of Denial cell
a. oxidation of zinc ions take place
b. oxidation of copper ions take place
c. oxidation of zinc take place
d. oxidation of copper take place
Question 8 : In Denial cell direction of electron flow is
a. toward cathode b. toward anode c. toward zinc electrode d. from copper to zinc electrode
Question 9 : Daniell cell has the emf value.
a. 1.09 V b.0.19 V c.9.01 V d. 10.9 V
Question 10 : Consider the reaction, 2Ag+ + Cd → 2Ag + Cd2+
The standard electrode potentials for Ag+ → Ag and Cd2+→ Cd couples are 0.80 volt and -0.40 volt, respectively. What is the standard potential DEo for this reaction?
a. 1.20 V b. 12.0 V c. 120 V d. 0.12 V
Question 11 : What will be the emf of the following cell Zn|Zn2+(aq)(1.0M)||Cu2+(aq)(1.0M)|Cu
The standard electrode potentials are: Cu2+ + 2e- → Cu(aq) Eo = 0.350 volt
Zn2+ + 2e- → Zn(aq) Eo = -0.763 volt
a. 1.113 V b. 12.43 V c. 1.92 V d. 5.324 V
Question 12 : What will be the electrode potential at a copper electrode dipped in a 0.1 M solution of copper sulphate at 25oC? The standard electrode potential of Cu2+/Cu system is 0.34 volt at 298 K.
a. 10.113 V b. 0.31045 V c. 11.92 V d. 9.324 V
Question 14: For a concentration cell E° =
a. 0 V b. 1 V c. 2 V d. 3 V
Question 15: In concentration cells EMF arises due to transfer of:
a. electrons b. charge c. matter d. protons
Дата добавления: 2018-09-20; просмотров: 323; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
