Free metal A Salt of metal B Salt of metal A Free metal B
Or, in net ionic form, MA + MB+ ®MA+ + MB
PROCEDURE:
1 Select four 100 ml beakers and label them as zinc sulphate, copper sulphate, aluminium sulphate and ferrous sulphate.
2 Add 20ml of 1M solution of ZnSO4, CuSO4, Al2(SO4)3 and FeSO4 in the labelled beaker respectively.
3 Take a strip of cleaned zinc metal and cut it into small pieces of suitable size.
4 Add two pieces into each beaker containing CuSO4, Al2(SO4)3 and FeSO4.
5 Keep the beakers undisturbed for about 1 hour.
6 After about an hour, note the change in colour of solutions, appearance of metal surface or any other changes.
7 Write an equation for the reaction between metal and salt.
8 Repeat the similar procedure by adding –
· Copper strips to ZnSO4, Al2(SO4)3 and FeSO4 solutions.
· Aluminium strips to ZnSO4, CuSO4, and FeSO4 solutions.
· Iron strips to ZnSO4, CuSO4 and Al2(SO4)3 solutions.
Observations:
| Metal | Salt solution in which added | Colour change of solution | Appearance of metal surface | Inference |
| Zinc | CuSO4 | Blue color of CuSO4 disappears and red-brown copper particles settle down at the bottom of the beaker. | Changes | Zn can displace Cu from CuSO4 solution. Zn + CuSO4 ---> ZnSO4 + Cu |
| Al2(SO4)3 | Solution remains colorless. | No change | Zn cannot displace Al from Al2(SO4)3 solution. | |
| FeSO4 | Green colour of ferrous sulphate disappears. Iron metal is settling down at the bottom of the beaker. | Changes | Zn can displace Fe from FeSO4 solution. Zn + FeSO4 ---> ZnSO4 + Fe | |
| Copper | ZnSO4 | Solution remains colorless. | No change | Cu cannot displace Zn from ZnSO4 solution. |
| Al2(SO4)3 | Solution remains colorless. | No change | Cu cannot displace Al from Al2(SO4)3 solution. | |
| FeSO4 | Solution remains colorless. | No change | Cu cannot displace Fe from FeSO4 solution. | |
| Aluminium | ZnSO4 | Solution remains colorless. Zinc metal is settling down at the bottom of the beaker. | Changes | Al can displace Zn from ZnSO4 solution. 2Al + 3ZnSO4 ---> 3Zn + Al2(SO4)3 |
| CuSO4 | Blue colour of CuSO4 disappears. The brown colored copper particles settle down at the bottom of the beaker. | Changes | Al can displace Cu from CuSO4 solution. 2Al + 3CuSO4 ---> 3Cu + Al2(SO4)3 | |
| FeSO4 | Green colour of FeSO4 disappears. Iron metal is settling down at the bottom of the beaker. | Changes | Al can displace Fe from FeSO4 solution. 2Al + 3FeSO4---> 3Fe + Al2(SO4)3 | |
| Iron | ZnSO4 | Solution remains colorless. | No change | Fe cannot displace Zn from ZnSO4 solution |
| CuSO4 | Blue colour of CuSO4 changes to light green colored FeSO4. Copper metal is formed in the beaker. | Changes | Fe can displace Cu from CuSO4 solution. Fe + CuSO4 -=> Cu + FeSO4 | |
| Al2(SO4)3 | Solution remains colorless. | No change | Fe cannot displace Al from Al2(SO4)3 solution. |
Conclusions:
· Aluminium is able to displace Zn, Cu and Fe from their salt solutions.
· Zinc is able to displace Cu and Fe from their salt solutions.
· Fe is able to displace Cu from its salt solution.
· Cu cannot displace Zn, Al or Fe from their salt solutions.
· Thus, aluminium is a more reactive metal. The reactivity of given metals in decreasing order is as given below:
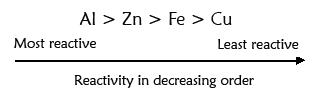
Perform test:
| № | Question | Variant |
| 1 | Na+ and K+ elements have: | a) tendency to gain electrons. b) positive reduction potential. c) negative reduction potential. d) negative oxidation potential. |
| 2 | Ca and Cu metals when coupled together gives maximum emf for a voltaic cell because: | a) It shows greater difference in standard reduction potential values. b) It shows less difference in standard reduction potential value. c) It shows same standard reduction potential value. d) None of these. |
| 3 | If half cell reaction A + e- ? A- has large negative reduction potential, it shows that: | a) A is readily reduced b) A- is readily reduced. c) A- is readily oxidized. d) None of these. |
| 4 | Four alkali metals K, L, M and N are having standard electrode potentials as -4.05, -2.66, -0.50 and 0.70 V respectively. Which one of the following is the most reducing? | a) N b) M c) L d) K |
| 5 | The oxidation potential of Mg and Al are +2.37 and +1.66 volt respectively. The Mg in chemical reaction: | a) will not replace Al at all. b) will replace Al. c) will be replaced by Al d) none of the above. |
| 6 | Three metal cations A, B and C are having standard reduction potential values as 0.73, -2.17 and -1.15 V respectively. The order of reducing power of corresponding metals is: | a) C > A > B b) A > B > C c) B > C > A d) C > B > A |
| 7 | Use the activity series given to you to determine which of the following reactions will NOT take place: | a) Zn + HNO3 --> b) Ag + AuCl3--> c) Ca + Pb(NO3)2--> d) Cu + HCl--> |
| 8 | Zn(s) => Zn2+(aq) + 2e The chemical equation above shows a corrosive half-reaction for zinc. Which of these best represents what is occurring? | a) Zinc is reduced and is acting as the oxidizing agent. b) Zinc is oxidized and is losing electrons. c) Zinc is reduced and is losing electrons. d) Zinc is oxidized and is gaining electrons. |
| 9 | Corrosion, a type of redox reaction, can cause cars and buildings to rust. Rusting happens when iron is oxidized by oxygen in the air. This process happens when …... | a) oxygen acts as the reducing agent and gains electrons b) iron acts as the reducing agent and loses electrons c) oxygen acts as the oxidizing agent and loses electrons d) iron acts as the oxidizing agent and gains electrons |
| 10 | In order to develop building materials that are resistant to corrosion, manufacturers must be aware of ways to promote… | a) anodic inhibition, which prevents the reduction of the metal b) cathodic reactions, which allow reduction of the metal c) anodic inhibition, which prevents the oxidation of the metal d) cathodic reactions, which allows oxidation of the metal |
| 11 | Some silverware is not made entirely of silver but is electroplated, or coated, with silver. The process of electroplating a fork would involve the migration of . | a) silver ions oxidized at the anode and depositing on the fork, which acts as the cathode b) ions oxidized from the fork (cathode) and depositing on the silver metal (anode) c) silver ions reduced at the anode and depositing on the fork, which acts as the cathode d) ions oxidized from the fork (anode) and depositing on the silver metal (cathode) |
| 12 | Which of the following is true about metals? | a) Irregular order of arrangement of atoms b) Metallic luster c) Bad conductors of electricity d) Low boiling point |
| 13 | On the basis of sequence of reactions, identify the most and least reactive elements: A + BX → AX + B, C + AY → CY + A | a) A, C b) C, A c) B, C d) C, B |
| 14 | Which one of the following metals does not displace Ag from a solution of AgNO3? | a) Au b) K c) Ca d) Cu |
| 15 | Name a least reactive metal. | a) Sn b) Na c) Ca d) Pt |
| 16 | If iron filings are put in different test tubes containing ZnSO₄, CuSO₄, Al₂(SO₄)3, CaCl₂, in which one changes will be observed? | a) ZnSO₄ b) CuSO₄ c) CaCl₂ d) Al₂(SO₄)3 |
| 17 | What is the correct decreasing order of the reactivity of the metals based on the reactions given below? Fe(s) + Pb(NO₃)₂(aq) --> Fe(NO₃)₂(aq)+ Pb(s) , Cu(s) + 2AgNO₃(aq) --> Cu(NO₃)₂(aq) + 2Ag(s) , Zn(s) + FeSO₄(aq) --> ZnSO₄(aq) + Fe(s) , Pb(s) + CuCl₂(aq) --> PbCl₂(aq) + Cu(s) | a) Zn > Fe > Pb > Cu > Ag b) Pb > Zn > Fe > Cu > Ag c) Fe > Zn > Cu > Ag > Pb d) Ag > Cu > Pb > Fe > Zn |
| 18 | Which one of the following is true about displacement reaction? | a) One component of both the reacting molecules gets exchanged to form the product. b) A single compound splits into two or more simple substances. c) Highly reactive metal replaces the least reactive one from its salt solution. d) Only a single product is formed. |
| 19 | Some crystals of copper sulphate were dissolved in water. What colour would be the solution obtained? | a) Green b) Blue c) Red d) Brown |
| 20 | Identify the set up in which reaction occurs. | a) FeSO4 + Cu => b) Ag + HCl => c) Al + CuSO4 = > d) H2 + CaO => |
| 21 | A student puts one big iron nail in each of four test tubes containing solutions of zinc sulphate, aluminium sulphate, copper sulphate and iron sulphate. A reddish brown coating was observed only on the surface of the iron nail which was put in which solution? | a) Zinc sulphate b) Copper sulphate c) Iron sulphate d) Aluminium sulphate |
| 22 | Iron nails were dipped in a solution kept in a test tube. After half an hour, it was observed that the colour of the solution had changed. Which solution was in the test tube? | a) Zinc sulphate b) Iron sulphate c) Copper sulphate d) Aluminium sulphate |
| 23 | When an aluminium strip is kept immersed in freshly prepared ferrous sulphate solution taken in a test tube, what change is observed? | a) The green solution slowly turns brown. b) The green solution turns colourless. c) A colourless gas with smell of burning sulphur is observed. d) Light green solution changes to blue. |
| 24 | Which of the following statements is not correct for the following reaction ? Cu + ZnSO₄ → CuSO₄ +Zn? | a) The reaction does not occur. b) Cu is less reactive than Zn. c) The colourless solution is ZnSO₄. d) Zn is less reactive than Cu. |
| 25 | Four test tubes were taken and marked A, B, C and D respectively. 2 mL of solution of Al₂(SO₄)₃ in water was filled in each of the four test tubes. Clean piece of metal Zinc was placed in test tube A, clean iron nail was put in test tube B, clean copper wire was placed in test tube C and a clean aluminium wire was placed in test tube D. It was observed that no change occurred in any of the test tubes. What is the correct inference? | a) Zinc is more active than Aluminium. b) Zinc is more active than Copper. c) Copper is more active than Aluminium. d) Zinc, Iron and copper are less active than Aluminium. |
| 26 | In which case will not a chemical reaction take place? | a) Zinc metal and copper sulphate solution b) Copper metal and copper sulphate solution c) Iron metal and copper sulphate solution d) Aluminium metal and copper sulphate solution |
| 27 | What is the correct procedure to show that zinc metal is more reactive than copper? | a) Prepare copper sulphate solution and dip zinc strip into it. b) Prepare zinc sulphate solution and dip a copper strip into it. c) Heat zinc and copper strips. d) Add dilute nitric acid on both strips. |
| 28 | A strip of copper was placed in zinc sulphate solution in a beaker. What was seen while observing on the next day? | a) Copper strip was unchanged. b) Copper strip became thinner. c) Copper strip became thicker. d) Colour of copper strip changed. |
| 29 | In which case does a chemical reaction take place? | a) Zinc metal and zinc sulphate solution b) Aluminium metal and zinc sulphate solution c) Iron metal and zinc sulphate solution d) Copper metal and zinc sulphate solution |
LABORATORY WORK
METAL CORROSION
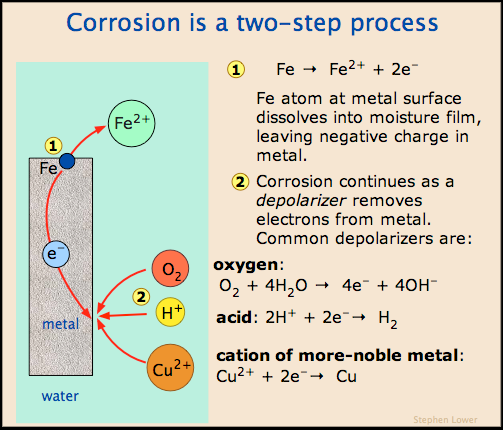
Objectives: Students will be able to observe and record the corrosive nature of oxidation-reduction reactions and to determine the electro-chemical series of selected metals (relative strengths of oxidizing and reducing agents).
Corrosion can be defined as the degradation of a material when it comes in contact with the environment.
The dissolving of a material by a corrosive liquid is called chemical corrosion. The material continues to dissolve until either it is consumed or the liquid is saturated. A simple example is salt dissolving in water.
The removing of metal atoms from a solid material as the result of an electric circuit is called electrochemical corrosion. In this form of corrosion, metal atoms lose electrons and thus become ions forming a byproduct. Electrochemical corrosion occurs most frequently in aqueous mediums, in which ions are present in water or moist air. In this process, an electric circuit is created and the system is called an electrochemical cell. Corrosion of a steel pipe or a steel automobile panel, creating holes in the steel and rust as the byproduct, are examples of this reaction.
Corrosion:
·  Silver articles become black after some time when exposed to air. This is because it reacts with sulphur in the air to form a coating of silver sulphide.
Silver articles become black after some time when exposed to air. This is because it reacts with sulphur in the air to form a coating of silver sulphide.
· Copper reacts with moist carbon dioxide in the air and slowly loses its shiny brown surface and gains a green coat. This green substance is copper carbonate.
· Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance called rust.
Prevention of Corrosion:
· The rusting of iron can be prevented by painting, oiling, greasing, galvanising, chrome plating, anodising or making alloys.
· Galvanisation is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc. The galvanised article is protected against rusting even if the zinc coating is broken.
· Alloying is a very good method of improving the properties of a metal. An alloy is a homogeneous mixture of two or more metals, or a metal and a nonmetal. It is prepared by first melting the primary metal, and then, dissolving the other elements in it in definite proportions. It is then cooled to room temperature. We can get the desired properties by this method.
· For example, iron is the most widely used metal. But it is never used in its pure state. This is because pure iron is very soft and stretches easily when hot. But, if it is mixed with a small amount of carbon (about 0.05 %), it becomes hard and strong.
· When iron is mixed with nickel and chromium, we get stainless steel, which is hard and does not rust. Thus, if iron is mixed with some other substance, its properties change.
Rusting of metals is a special case of metal oxidation. Iron will oxidize to form rust. Water will cause metals to rust; this reaction can be accelerated by adding salts. In the corrosion process, metals get oxidized. For example in mild steel (which is greater than 99% iron) the metal corrodes according to the following:
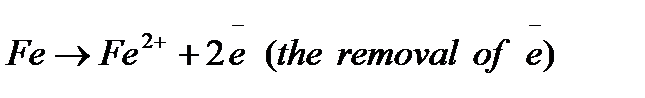
These electrons are consumed by reacting with another substance (usually oxygen but it can be H+ in acids) in reduction as in
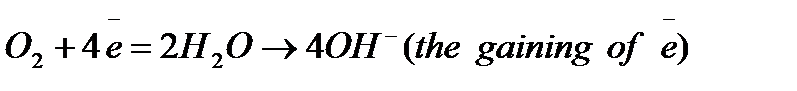
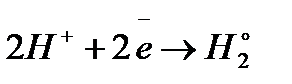
In an acid solution, the reduction is:
These equations indicate that in order for metals to corrode (rust), two reactions occur; an oxidation that converts metal to metal ions and electrons and a second reaction which consumes those electrons by converting oxygen and water to hydroxide ions. In order for these reactions to occur, the electrons must be transported from the place where the metal dissolves to the place where the oxygen is consumed and an ionic current must also flow between the sites to complete the circuit. This ionic current flows more easily through water containing electrolytes (i.e., NaCl). This accounts for the rapid rusting of unprotected steel in a salty environment.
The final product of iron oxidation (rust) is usually a ferric oxide (often hematite Fe2O3). The initial corrosion product of the anodic reaction is ferrous (Fe2+) ion. This is subsequently oxidized to Fe3+ by exposure to oxygen.
Hydrogen ions are being consumed by the process. As the iron corrodes, the pH in the droplet rises. Hydroxide ions (OH-) appear in water as the hydrogen ion concentration falls. They react with the iron (II) ions to produce insoluble iron (II) hydroxides or green rust:
Fe2+(aq) + 2OH-(aq) --> Fe(OH)2(s)
The iron (II) ions also react with hydrogen ions and oxygen to produce iron (III) ions:
4Fe2+(aq) + 4H+(aq) + O2(aq) -->4Fe3+ (aq) + 2H2O(l)
The iron (III) ions react with hydroxide ions to produce hydrated iron(III) oxides (also known as iron (III) hydroxides):
Fe3+ (aq) + 3OH- (aq) --> Fe(OH)3 (s)
The loose porous rust or Fe(OH)3 can slowly transform into a crystallized form written as Fe2O3.H2O the familiar red-brown stuff that is called "rust" forming tubercles as shown here. Since these processes involve hydrogen ions or hydroxide ions, they will be affected by changes in pH. With limited O2, magnetite is formed (Fe3O4).
The corrosion process may be slowed by coating the metals with other metals or polymers in order to protect the metal from the corrosive environment. Examples of this can be seen in food cans which have a polymer coating and in galvanized steel where iron is coated with zinc.
When we put two metals in direct contact, one can oxidize (rust) while the other reduces oxygen. This reaction sets up a voltage and is the primary reaction in a battery. By measuring this voltage, it is possible to construct a list ranking the metal's oxidation tendencies. If metals which are far apart in oxidation tendencies are placed in contact with each other and with an electrolyte solution, severe corrosion of one metal can occur.
1st Experiment. Rusting of iron.
Only Iron and steel rust. Other metals corrode. Rusting is an oxidation process. What we normally call rust is a flaky red-brown solid which is largely hydrated iron?
The primary corrosion product of iron is Fe(OH)2 (or more likely FeO*nH2O), but the action of oxygen and water can yield other products having different colors:
· Fe2O3*H2O (hydrous ferrous oxide, sometimes written as Fe(OH)3) is the principal component of red-brown rust. It can form a mineral called hematite.
· Fe3O4*H2O ("hydrated magnetite" or ferrous ferrite, Fe2O3*FeO) is most often green but can be deep blue in the presence of organic complexants as shown here.
· Fe3O4 ("magnetite") is black.
Materials and reagents: test tubes, water, 12% acetic acid solution (CH3COOH) or vinegar, sodium hydroxide (NaOH) concentrated solution, nails.
Procedure:
1) For the first experiment put a 5 cm finishing nail brushed with steel wool in a test tube containing 8 mL of tap water. The picture was taken three days later.
2) The second experiment was carried out with an acidic solution containing 12% acetic acid. The second picture was taken an hour after the start of the experiment. You can produce a similar reaction with vinegar which typically contains 3% acetic acid in water. However, you will have to be more patient than we were.
3) In the third experiment a pellet of sodium hydroxide (caustic 'Drano') was dropped in the test tube of the second experiment. The test tube was capped to prevent air to react with the solution. After three hours blue rust precipitates had more or less settled out as you can see in this picture:
| 1 | 2 | 3 |
| Red-brown flaky rust | Black magnetite | Blue/green unstable rust |

| 
| 
|
| Formula | Color | Oxidation State | Structure / comments |
| Fe2O3*H2O or Fe(OH)3 | red brown | Fe3+ | a-form Hematite, b-form used in recording tapes |
| Fe3O4 | black | Fe2+ and Fe3+ | Magnetite / lodestone |
| Fe(OH)2 | blue/green | Fe2+ | Soluble, the color going from yellow to green and blue by changing the pH of the solution from acidic to very basic |
| FeO | black | Fe2+ | Pyrophoric |
2nd Experiment. Cathodic Protection
This is a little corrosion experiment where we will show how Cathodic Protection works and how is possible to protect a metal, an iron nail in this case, from corrosion using the principle of Galvanic Corrosion.
The same principle is used to protect Ship and Offshore structure like Oil&Gas Platform from Corrosion.
This experiment shows also how nails rust when are exposed to water. What we will do to expose two identical iron nails to the same electrolyte, in this case fresh water with two spoons of NaCl, salt that you use for cooking. One of the nails will be connected through an electrical wire to a piece of aluminium foil, the same will be used for cooking, while the second nail will be immersed in the same electrolyte.
We will leave the two nails for two days immersed in water and then we will see if Cathodic Protection has protected the first nail from corrosion.
Materials and reagents: two identical iron nails; a piece of aluminium foil; an electrical wire; something to hang the second nail, we've used an elastic a glass; two spoons of salt, NaCl, the same that you use for cooking
 Procedure:
Procedure:

In the 1 and 2 pictures below you can see how we've connected the nail to the cable and to the aluminium foil. To fix the aluminum foil just wrap it around the electrical cable and be sure that there is a contact between the aluminum and copper.
 You can see how we have immersed the nails. This picture has been taken after two days of exposure. The nail that is not connected to aluminium is corroded.
You can see how we have immersed the nails. This picture has been taken after two days of exposure. The nail that is not connected to aluminium is corroded.

Fig 4 - On the left you can see the nail without cathodic protection, in the middle the nail connected to the aluminium foil, cathodically protected, on the right a new nail.

You can see how the nail without cathodic protection, that is not connected to the aluminium is severely corroded, see the red rust, while the second nail shown only minor sign of corrosion.

You can see the red rust, iron oxide (fig. 6).
In this experiment we've shown how cathodic protection works. When you connect a metal, in this case aluminium, that in the Galvanic series of metals is below Iron, has a lower potential and is anodic, corrodes and protect Iron from Corrosion. The nail that is not connected to aluminium will corrode severely only after two days of exposure.
The amount of corrosion is a function of several factors, like the temperature of water, the amount of salt dissolved, the chemical composition of the nail. In this case as you can see in the picture, the nails are galvanized, it means that they have a thin superficial layer of zinc deposited on the surface, and this gives greater resistance to corrosion compared to steel. If we use a nail that is not galvanized it corrodes faster.
Perform test:
| № | Question | Variant |
| 1 | Identify the correct statement | a) Rusting is the oxidation of any metal b) Corrosion is the oxidation of any metal with rusting being about iron only c) Rusting is the oxidation of any metal |
| 2 | When iron rusts, which of these ion-electron equations is correct? | a) Fe(s) → Fe(aq) b) Fe2+(aq) + 2e → Fe(s) c) Fe(s) → Fe2+ + 2e- |
| 3 | What is the colour change that Ferroxyl indicator shows when iron is oxidised to ions? | a) Yellow → pink b) Blue → yellow c) Yellow → blue |
| 4 | A motoring organisation noticed that cars rust faster when owned by people who live by the sea in comparison to people who live inland. What is the main reason for this? | a) Sea water is a salt solution full of ions b) The wind increases the concentration of oxygen at the seaside c) Seagull droppings are acidic |
| 5 | How does salt speed corrosion? | a) Salt contains oxygen b) Salt is an electrolyte and can carry charged particles c) Salt is sodium chloride and sodium is reactive |
| 6 | Elise investigated rusting by attaching steel to a car battery. Which is the true statement? | a) Attaching steel to the negative terminal of a battery protects it from rusting b) Attaching steel to the positive terminal of a battery protects it from rusting c) Attaching steel to a battery has no effect on rusting |
| 7 | Identify the oxidation equation involved in corrosion. | a) Fe2+(aq) + 2e- → Fe(s) b) Fe2+(aq) → Fe3+(aq) + e- c) 2H2O(l) + O2(g) + 4e- → 4OH-(aq) |
| 8 | Identify the reduction equation involved in corrosion. | a) Fe2+(aq) + 2e- → Fe(s) b) Fe2+(aq) → Fe3+(aq) + e- c) 2H2O(l) + O2(g) + 4e- → 4OH-(aq) |
| 9 | Magnesium metal can be attached to the steel pipeline to prevent rusting. What name is given to this type of protection provided by magnesium? | a) Galvanising b) Sacrificial c) Anodising |
| 10 | Steel cars can be protected from rusting in a number of ways. Dipping steel in molten zinc can also physically protect steel from rust. Why would the steel not rust even if the zinc is scratched? | a) The molten zinc will fill in the scratch b) Zinc is less reactive than steel and will give electrons to steel c) Zinc is more reactive than steel and will give electrons to steel |
| 11 | Which of these metal elements has to be stored under oil? | a) mercury b) sodium c) aluminium |
| 12 | Which of these metal elements produces hydrogen gas when it is placed in hydrochloric acid? | a) iron b) copper c) silver |
| 13 | Which of these metal elements will form an alkali when dropped into cold water? | a) lead b) aluminium c) calcium |
| 14 | Which of the following groups of 4 metals is arranged in descending order of their chemical reactivity? | a) potassium, magnesium, lead, iron b) sodium, aluminium, tin, gold c) calcium, zinc, aluminium, silver |
| 15 | Which of these sets of elements contain metals all known to mankind in prehistoric times? | a) aluminium, silver and mercury b) mercury, silver and gold c) calcium, copper and tin |
| 16 | In which of the following pairs of metal elements are both easily extracted from their ores by heating with carbon? | a) tin and copper b) magnesium and silver c) zinc and potassium |
| 17 | When sodium reacts with water the two products of the reaction are: | a) sodium oxide and hydrogen b) sodium hydroxide and oxygen c) sodium hydroxide and hydrogen |
| 18 | Which of the following shows two metals which will both react quickly with cold water? | a) aluminium and calcium b) potassium and sodium c) sodium and magnesium |
| 19 | Identify the pair of substances which would react together to give a gas as one of their products? | a) iron and dilute sulphuric acid b) copper and water c) magnesium and oxygen |
| 20 | Choose the pair of metal elements where both are required to be extracted from their ores by means of electrolysis. | a) copper and zinc b) magnesium and iron c) potassium and aluminium |
QUESTIONS FOR THE EXAM
Elements of Chemical Thermodynamics
1. Subject and tasks of chemical thermodynamics. Chemical thermodynamics as the basis of bioenergetics. Isolated, closed and open systems.
2. The first law of thermodynamics. Internal energy, heat and work. Isobaric and isochoric thermal processes. Enthalpy.
3. Hess’s law and its corollaries. Standard formation and combustion heat. Thermochemical calculations and their usage for energetic characteristic of biochemical processes.
4. Interconnection between the processes of metabolism and energy exchange. Caloric value of main constituents of food and some food products. Energy consumption at different modes of moving activity.
5. Thermodynamically reversible and irreversible processes. The second law of thermodynamics. Entropy. Statistic and thermodynamic explanation of entropy. Standard entropy.
6. The Gibbs free energy (isobaric-isothermal potential). Enthalpy and entropy factors. Endothermic and exothermic processes.
7. Thermodynamics of chemical equilibrium. Reversible and irreversible reactions. Concept of chemical equilibrium. Constant of the chemical equilibrium. The interconnection between the constant of chemical equilibrium and the Gibbs free energy. Equations of isotherm and isobaric curve of a chemical reaction.
Elements of Chemical Kinetics
8. Main concepts of chemical kinetics. Simple and complex, homogeneous and heterogeneous reactions. The speed of homogeneous chemical reactions and methods of its measuring.
9. The main postulate of chemical kinetics. The order of reaction and the reaction speed constant. The Law of mass action for the speed of the reaction and its sphere of application.
10. Kinetic equations of the reactions of zero, first and second order. Period of semi-transformation. Molecularity of the reaction.
11. Theory of active collisions. Arrhenius’ equation. Energy of activation. Vant-Hoff’s rule. Temperature coefficient of the reaction speed for enzymatic processes.
12. Catalysis and catalysts. The theories of catalysis. The mechanism of homogeneous and heterogeneous catalysis. Enzymes as biological catalysts, peculiarities of their action.
Colligative Properties of Solutions
13. Thermodynamics of solution formation.
14. Osmose and osmotic pressure of solutions. Vant-Hoff’s law.
15. Osmotic pressure, osmolarity and osmolality of some biological fluids. The concept of isotonic, hypertonic and hypotonic solutions.
16. The role of osmotic phenomena in biological processes.
17. The pressure of saturated vapor of solvent above the solution. Raoul’s first law.
18. Boiling and freezing temperatures of solvents. Raoul’s second law. Cryoscopy. Ebullioscopy.
19. Colligative properties of electrolyte solutions. Isotonic coefficient.
Electrolyte solutions. Acidity and basicity of aqueous solutions, pH.
20. The theory of weak electrolyte solutions. Main characteristics of a weak electrolyte: pH, Кion, рКion.
21. The theory of strong electrolyte solutions. Main characteristics of a strong electrolyte: а, fa, I.
22. Protolytic theory of acids and bases.
23. The ion product of water. Hydrogen ion exponent pH.
24. Calculation of solution pH of weak and strong acids and bases.
25. Determination of hydrogen ion exponent.
26. Role of hydrogen ions in biological processes.
27. Buffer systems, their classifications.
28. Calculation of рН of acid and basic buffer solutions.
29. Mechanism of action of buffer systems.
30. Buffer capacity.
Atomic Properties and Periodic Trends
31. The electron shell of the atom. Energy levels. The wave nature of microparticles motion. The uncertainty principle. The electron cloud.
32. The quantum numbers. The principal quantum number n. The angular momentum quantum number ℓ. Magnetic quantum number mℓ . Electron spin. The shapes of atomic orbitals.
33. Electron configurations of the elements. The Pauly principle. Hund's rule. Order of orbital energies and assignments. Orbital energies. Electron configurations of the main group elements. Electron configurations of the transition elements.
34. The periodic table. Atomic properties and periodic trends. Atomic size. Ionization energy. Electron affinity.
Chemical Bond
35. Chemical bond formation.Valence electrons. The formation of a covalent bond in H2 molecule.
36. Properties of covalent bond. The bond order. Single and multiple bonds. Sigma and π-bond. The donor–accepter mechanism of formation of covalent bond. Bond length. Bond energy. 60. Polarity and electronegativity. Oxidation numbers. Ionic bond.
37. Orbital hybridization. Hybrid orbitals. Molecular Shape. Molecular polarity.
38. Orbital hybridization in CH4 , H2O and NH3 molecules.
39. Theories of chemical bonding. The valence bond (VB) theory and the molecular orbital (MO) theory. Molecular orbital theory: principles of molecular orbital theory, bonding and antibonding molecular orbitals, bond order. Electron configurations for H2 molecule.
Complex (Coodination) Compounds
40. The structure of complex compounds: inner sphere, outer sphere, central atom, ligands, coodination number, dentation of ligands.
41. The nature of the chemical bond in complex compounds.
42. Classification and nomenclature of complex compounds. Cyclic complexes or chelates.
43. Dissociation of complex compounds in solutions. Destruction of complex compounds.
44. Equilibria and the processes in solutions with complex compounds.
Dispersion Systems
45. Dispersion systems, their peculiarities and classification.
46. Molecular-kinetic properties of colloidal systems. Sedimentation.
47. Optical properties of colloidal systems . Opalescence.
48. Structure of colloid particles.
49. Methods of obtaining and purification of colloidal systems. Peptization.
50. Colloid protection and its importance.
GLOSSARY
Acid - a compound that, when dissolved in water, gives a pH of less than 7.0 or a compound that donates a hydrogen ion
anion - negatively charge ions
atom - a chemical element in its smallest form, and is made up of neutrons and protons within the nucleus and electrons circling the nucleus
atomic number - the number representing an element which corresponds with the number of protons within the nucleus
atomic orbital - the region where the electron of the atom may be found
absolute zero - Absolute zero is °K. It is the lowest possible temperature. Theoretically, at absolute zero, atoms stop moving.
accuracy - Accuracy is a measure of how close a measured value is to its true value. For example, if an object is exactly a meter long and you measure it as 1.1 meters long, that is more accurate than if you measured it at 1.5 meters long.
acid - There are several ways to define an acid, but they include any chemical that gives off protons or H+ in water. Acids have a pH less than 7. They turn the pH indicator phenophthalein colorless and turn litmus paper red.
acid anhydride - An acid anhydride is an oxide that forms an acid when it is reacted with water. For example, when SO3- is added to water, it becomes sulfuric acid, H2SO4.
actual yield - The actual yield is the amount of product you actually obtain from a chemical reaction, as in the amount you can measure or weigh as opposed to a calculated value.
addition reaction - An addition reaction is a chemical reaction in which atoms add to a carbon-carbon multiple bond.
alkali metal - An alkali metal is a metal in Group I of the periodic table. Examples of alkali metals include lithium, sodium, and potassium.
alkaline earth metal - An alkaline earth metal is an element belonging to Group II of the periodic table. Examples of alkaline earth metals are magnesium and calcium.
allotrope - Allotropes are different forms of a phase of an element. For example, diamond and graphite are allotropes of carbon.
alpha particle - An alpha particle is another name for a helium nucleus, which contains two protons and two neutrons. It's called an alpha particle in reference to radioactive (alpha) decay.
Base - a substance that accepts a proton and has a high pH; a common example is sodium hydroxide (NaOH)
boiling - the phase transition of liquid vaporizing
bond - the attraction and repulsion between atoms and molecules that is a cornerstone of chemistry
burette (also buret) - glassware used to dispense specific amounts of liquid when precision is necessary (e.g. titration and resource dependent reactions)
binary compound - A binary compound is one made up of two elements.
binding energy - Binding energy is the energy that holds protons and neutrons together in the atomic nucleus.
bond energy - Bond energy is the amount of energy required to break one mole of chemical bonds.
bond length - Bond length is the average distance between the nuclei of two atoms that share a bond.
buffer - A liquid that resists change in pH when an acid or base is added. A buffer consists of a weak acid and its conjugate base. An example of a buffer is acetic acid and sodium acetate.
Catalyst - a chemical compound used to change the rate (either to speed up or slow down) of a reaction, but is regenerated at the end of the reaction.
cation - positively charged ion
calorimetry - Calorimetry is the study of heat flow. Calorimetry may be used to find the heat of reaction of two compounds or the heat of combustion of a compound, for example.
cathode - A cathode is the electrode which gains electrons or is reduced. In other words, it is where reduction occurs in an electrochemical cell.
chemical equation - A chemical equation is a description of a chemical reaction, including what reacts, what is produced, and which direction(s) the reaction proceeds.
chemical property - A chemical property is a property that can only be observed when achemical change occurs. Flammability is an example of a chemical property, since you can't measure how flammable a substance is without igniting it (making/breaking chemical bonds).
covalent bond - A covalent bond is a chemical bond formed when two atoms share two electrons.
critical mass - Critical mass is the minimum quantity of radioactive material needed to cause a nuclear chain reaction.
critical point - The critical point is the endpoint of the liquid-vapor line in a phase diagram, past which a supercritical liquid forms. At the critical point, the liquid and vapor phases become indistinguishable from one another.
crystal - A crystal is an ordered, repeating three-dimensional pattern of ions, atoms, or molecules. Most crystals are ionic solids, although other forms of crystals exist.
centrifuge - equipment used to separate substances based on density by rotating the tubes around a centred axis
cell potential - the force in a galvanic cell that pulls electron through reducing agent to oxidizing agent
chemical Law - certain rules that pertain to the laws of nature and chemistry – examples
chemical reaction - the change of one or more substances into another or multiple substances
colloid - mixture of evenly dispersed substances, such as many milks
compound - a substance that is made up of two or more chemically bonded elements
condensation - the phase change from gas to liquid
conductor - material that allows electric flow more freely
covalent bond - chemical bond that involves sharing electrons
crystal - a solid that is packed with ions, molecules or atoms in an orderly fashion
cuvette - glassware used in spectroscopic experiments. It is usually made of plastic, glass or quartz and should be as clean and clear as possible
Dissolution or solvation - the spread of ions in a solvent
double bond - sharing of two pairs of electrons
diffusion - Diffusion is the movement of particles from an area of higher concentration to one of lower concentration.
dilution - Dilution is when solvent is added to a solution, making it less concentrated.
dissociation - Dissociation is when a chemical reaction breaks a compound into two or more parts. For example, NaCl dissociates into Na+ and Cl- in water.
double displacement reaction - A double displacement or double replacement reaction is when cations of two compounds switch places.
Earth metal - see alkaline earth metal
electrolyte - a solution that conducts a certain amount of current and can be split
electrochemical cell - using a chemical reaction's current, electromotive force is made
electron - a subatomic particle with a net charge that is negative
electron shells - an orbital around the atom's nucleus that has a fixed number electric charge - a measured property (coulombs) that determine electromagnetic interaction
element - an atom that is defined by its atomic number
energy - A system's ability to do work
enthalpy - Enthalpy is a measure of the amount of energy in a system (usually symbolized as H)
entropy - Entropy is a measure of the disorder or randomness in a system (usually symbolized as S)
electrolysis - Electrolysis is using electricity to break the bonds in a compound to break it apart.
electrolyte - An electrolyte is an ionic compound that dissolves in water to produce ions, which can conduct electricity. Strong electrolytes completely dissociate in water, while weak electrolytes only partially dissociate or break apart in water.
Faraday constant - a unit of electrical charge widely used in electrochemistry and equal to ~ 96,500 coulombs. It represents 1 mol of electrons, or the Avogadro number of electrons: 6.022 × 1023 electrons. F = 96 485.339 9(24) C/mol
Faraday's law of electrolysis - a two part law that Michael Faraday published about electrolysis
freezing - phase transition from liquid to solid
Galvanic cell - battery made up of electrochemical with two different metals connected by salt bridge
gas - particles that fill their container though have no definite shape or volume
Gibbs energy - value that indicates the spontaneity of a reaction (usually symbolized as G)
Halogens - Group 17 on the Periodic Table and are all non-metals
heat - energy transferred from one system to another by thermal interaction
Iodium – Latin name of the halogen element iodine
indicator - a special compound added to solution that changes color depending on the acidity of th e solution; different indicators have different colors and effective pH ranges
inorganic compound - compounds that do not contain carbon, though there are exceptions (see main article)
inorganic chemistry - a part of chemistry concerned with inorganic compounds
ion - a molecule that has gained or lost one or more electrons
ionic bond - electrostatic attraction between oppositely charged ions
Kinetics - A sub-field of chemistry specializing in reaction rates
Kinetic energy - The energy of an object due to its motion.
Lanthanides - Elements 57 through 71
lattice - Unique arrangement of atoms or molecules in a crystalline liquid or solid.
liquid - A state of matter which takes the shape of its container
law of conservation of energy - The law of conservation of energy states the energy of the universe may change form, but its amount remains unchanged.
ligand - A ligand is a molecule or ion stuck to the central atom in a complex. Examples of common ligands include water, carbon monoxide, and ammonia.
Metal - Chemical element that is a good conductor of both electricity and heat and forms cations and ionic bonds with non-metals.
melting - The phase change from a solid to a liquid
metalloid - A substance possessing both the properties of metals and non-metals
microcentrifuge - a small plastic container that is used to store small amounts of liquid
mole - abbreviated mol - a measurement of an amount of substance; a single mole contains approximately 6.022×1023 units or entities
molecule - a chemically bonded number of atoms that are electrically neutral
molecular orbital - region where an electron can be found in a molecule (as opposed to an atom)
Neutron - a neutral unit or subatomic particle that has no net charge
nucleus - the centre of an atom made up of neutrons and protons, with a net positive charge
noble gases - group 18 elements, those whose outer electron shell is filled
non-metal - an element which is not metallic
number density – a measure of concentration of countable objects (atoms, molecules, etc.) in space; number per volume
Orbital - may refer to either an atomic orbital or a molecular orbital
organic compound - compounds that contain carbon
organic chemistry - a part of chemistry concerned with organic compounds
oxidation number is the apparent charge on an atom. For example, the oxidation number of an oxygen atom is -2.
Poor metal - Metallic elements in the p-block, characterized by lower melting and boiling points than other metals
proton - a positive unit or subatomic particle that has a positive charge
protonation - the addition of a proton (H+) to an atom, molecule, or ion
pH - the measure of acidity (or basicity) of a solution
radioactivity - Radioactivity occurs when the atomic nucleus is unstable and breaks apart, releasing energy or radiation.
Raoult's Law - Raoult's Law states that the vapor pressure of a solution is directly proportional to the mole fraction of solvent.
rate determining step - The rate determining step is the slowest step in any chemical reaction.
rate law - A rate law is a mathematical expression relating the speed of a chemical reaction as a function of concentration.
redox reaction - A redox reaction is a chemical reaction that involves oxidation and reduction.
Salts - ionic compounds composed of anions and cations.
salt bridge - devices used to connection reduction with oxidation half-cells in an electrochemical cell.
saline solution - general term for NaCl in water.
s-block elements - Group 1 and 2 elements (alkali and alkaline metals), which includes Hydrogen and Helium.
single bond - sharing one pair of electrons.
solid - one of the states of matter, where the molecules are packed close together, there is a resistance of movement/deformation and volume change.
solution - homogeneous mixture made up of multiple substances. It is made up of solutes and solvents.
solvent - the part of the solution that dissolves the solute (H2O in saline water).
spectroscopy - study of radiation and matter, such as X-ray absorption and emission spectroscopy.
speed of light - the speed of anything that has zero rest mass (Energyrest = mc² where m is the mass and c is the speed of light).
Standard conditions for temperature and pressure or SATP - a standardisation used in order compare experimental results (25 °C and 100.000 kPa).
state of matter - matter having a homogeneous, macroscopic phase; gas, plasma, liquid, and solid are the most well known (in increasing concentration).
strong acid - A strong acid is an acid that completely dissociates in water. An example of a strong acid is hydrochloric acid, HCl, which dissociates into H+ and Cl- in water.
sublimation - Sublimation is when a solid changes directly into a gas. At atmospheric pressure, dry ice or solid carbon dioxide goes directly into carbon dioxide vapor, never becoming liquid carbon dioxide.
synthesis - synthesis is making a larger molecule from two or more atoms or smaller molecules.
system - system includes everything you are evaluating in a situation.
subatomic particles - particles that are smaller than an atom; examples are protons, neutrons and electrons.
substance - material with definite chemical composition.
Temperature - the average energy of microscopic motions of particles.
theory - a model describing the nature of a phenomenon.
thermochemistry - the study of absorption/release of heat within a chemical reaction.
thermodynamics - the study of the effects of changing temperature, volume or pressure (or work, heat, and energy) on a macroscopic scale.
thermodynamic stability - when a system is in its lowest energy state with its environment (equilibrium).
titration - the process of titrating one solution with another, also called volumetric analysis.
Tyndall effect - the effect of light scattering by colloidal (mixture where one substance is dispersed evenly through another) or suspended particles
Unit cell - the smallest repeating unit of a lattice.
universal or ideal gas constant - proportionality constant in the ideal gas law (0.08206 L·atm/(K·mol)).
Valence electron - the outermost electrons of an atom, which are located in electron shells.
Valence bond theory - a theory explaining the chemical bonding within molecules by discussing valencies, the number of chemical bonds formed by an atom.
van der Waals force - one of the forces (attraction/repulsion) between molecules.
Vapor - when a substance is below the critical temperature while in the gas phase.
vapour pressure - pressure of vapour over a liquid at equilibrium.
Water - H2O - a chemical substance, a major part of cells and Earth, and covalently bonded.
work - the amount of force over distance and is in terms of joules (energy)
X-ray - form of ionizing, electromagnetic radiation, between gamma and UV rays.
X-ray photoelectron spectroscopy - a spectroscopic technique to measure composition of a material.
Zone melting - a way to remove impurities from an element by melting it and slowly travel down an ingot (cast).
LITERATURE SOURCES
1 Chemistry (higher secondary, volume II. Tamilnadu. Textbook corporation. College Road, Chennai – 600 006). 2007.
2 Rob Lewis, Wynne Evans. Chemistry. Published by PALGRAVE MACMILLAN, NY, 2011.
3 Allan Blackman (University of Otago), Siegberd Schmio (University of Sydney). Chemistry.
4 Raymond Chang (William College), Kenneth A. Goldsby (Florida State University) Chemistry.
5 Glinka N.L. Collection of tasks and exercises of the general chemistry. - L.: Chemistry, 1987.
6 Standardized Test Practice. Student Edition. Glencoe Chemistry: Matter and Change. Printed in the United States of America. Glencoe/McGraw-Hill 8787 Orion Place. Columbus. 2010. Page 1-56.
7 J. Mc Murry and R. Fay "Chemistry" 3th ed. Prentice Hall, Upper Saddle River, New Jersey 07458, ISBN 0-13-087205-9. 2001.
8 Ebbing, D.D. and S.D. Gammon. 2002. General Chemistry. Seventh Edition. Houghton Mifflin Co., Boston, MA.
9 Maria Fenyes. Applied Chemistry. Chemistry 101. Laboratory Manual. Los Angeles. Mission College. 2008. Page 1 – 191.
Elmira Alimkulova
Nazira Mukhanbetova
| Was given to printing | Signed to printing 29.10.2015 |
| Format 60*84 1/16 | Order № 620 |
| Printed page 10,5 | 50 copies |
Publishing house of S.Seifullin Kazakh Agro Technical University. 2015
010011 62a Prospect Pobedy. Astana. Kazakhstan.
Дата добавления: 2018-09-20; просмотров: 334; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
