Read the text. Using a dictionary, translate it in writing.
Text A. Water. General Information
“Water is H 2O, hydrogen two parts, oxygen one, but there is also a third thing that makes water and nobody knows what that is.”
D.H. LAWRENCE (1885-1930)
Water is a substance composed of the chemical elements hydrogen and oxygen and existing on the Earth in all three physical states: solid, 8
 liquid, and gas. Water is a colourless, tasteless, and odourless liquid at room temperature. Its melting point is 0o C (32o F), and its boiling point is 100o C (212o F). Water is undoubtedly the most common, plentiful and essential of all chemical compounds.
liquid, and gas. Water is a colourless, tasteless, and odourless liquid at room temperature. Its melting point is 0o C (32o F), and its boiling point is 100o C (212o F). Water is undoubtedly the most common, plentiful and essential of all chemical compounds.
 Significance of Water for Life. Water is vital to life and essential to all living organisms. Life is believed to have originated in the world's oceans, so water has played a central role in the development of life on Earth. One of water’s most important properties is its ability to be a sol- vent for many other substances, which is essential to living organisms. They use aqueous solutions as a medium for carrying out biological pro- cesses. In fact, water participates in every process that occurs in plants and animals.
Significance of Water for Life. Water is vital to life and essential to all living organisms. Life is believed to have originated in the world's oceans, so water has played a central role in the development of life on Earth. One of water’s most important properties is its ability to be a sol- vent for many other substances, which is essential to living organisms. They use aqueous solutions as a medium for carrying out biological pro- cesses. In fact, water participates in every process that occurs in plants and animals.
Water Properties. Although the water molecule formula seems simple in structure (H2O), the physical and chemical properties of the compound are extremely complex. These proper- ties are incompletely understood and are not typical of most substances. For example, water can sometimes act as an acid or as an alkali (a base). Anoth- er unusual property is that in its solid form, ice, water is less dense than
when it is liquid. Ice therefore floats on water and protects the aquatic life below water surface of water bodies in cold areas of the world. Wa- ter occurs as a liquid on the surface of the Earth under normal conditions, which makes it invaluable for transportation, for recreation, and as a hab- itat for a myriad of plants and animals. The fact that water is readily changed to a vapour (gas) allows it to be transported through the atmos- phere from the oceans to inland areas where it condenses and, as rain, nourishes plant and animal life. The process is called the “water cycle”, or the “hydrologic cycle”.
Water Characteristics. Water quality is determined by assessing three classes of characteristics: physical, chemical, and biological. The physical characteristics include turbidity, colour, taste, odour, tempera- ture, and foamability. The chemical characteristics of water are its acidity, alkalinity, pH, hardness, and corrosiveness (corrosivity). The
|
|
|
9
 biological characteristics of a water body refer to a variety of living or- ganisms that can be found in water, including microscopic viruses, bacte- ria and protozoans, as well as phytoplankton (microscopic algae), zoo- plankton (tiny water animals), insects, worms, large plants and fish.
biological characteristics of a water body refer to a variety of living or- ganisms that can be found in water, including microscopic viruses, bacte- ria and protozoans, as well as phytoplankton (microscopic algae), zoo- plankton (tiny water animals), insects, worms, large plants and fish.
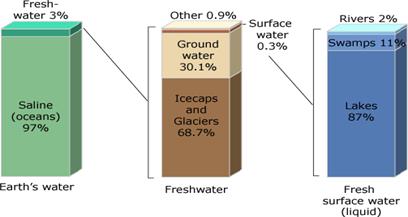
Earth’s Water Supply. About 97% of all water is salt (saline) water of the oceans, and the remaining 3% is fresh water. The majority of fresh water, about 69%, is locked up in polar glaciers and icecaps, main- ly of Greenland and Antarctica; and the rest is ground water. No matter where on Earth we stand, chances are that, at some depth, the ground below is saturated with water. Of all the fresh water on Earth, only about 0.3% is contained in rivers and lakes, known as surface water. Consider- ing that most of the water we use in everyday life comes from rivers, we make use of a tiny portion of the available water supplies.
 |
Дата добавления: 2019-02-22; просмотров: 363; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
