Study of chain transfer reactions to the polymer by spin trap.
In a series of works performed at the Research Institute Chemistry of Nizhny Novgorod State University them. NI Lobachevsky, an original approach to controlled synthesis of macromolecules under conditions of radical initiation, consisting in the generation of high-molecular nitroxide radicals - chain growth regulators directly into the the process of synthesis of macromolecules (in situ). As sources 26th nitroxide radicals were selected spin traps, in particular nitroso compounds and nitrones. A distinctive feature of these connections is the ability to react actively with short-lived radicals with the formation of stable spin-adducts. In particular, as effective regulators of the polymer lifetime chains for a number of vinyl monomers of different structures for the first time C-phenyl-N-t-butylnitron (PBN), 2-methyl-2- Nitrosopropane (MNP), nitrosodurol (ND) and other similar compounds that, as it turned out, can be effective regulating effect on the polymerization of a wide range of monomers in temperature range of 50-100 ° C. Thus, the use of PBN and MNP allows controlled polymerization of not only styrene, but also MMA, BA and butyl (meth) acrylate, vinyl acetate (VA) and vinyl chloride.The EPR method establishes that the spin trap accepts oligomeric radical with the formation of spatially hindered nitroxide radical (X •): 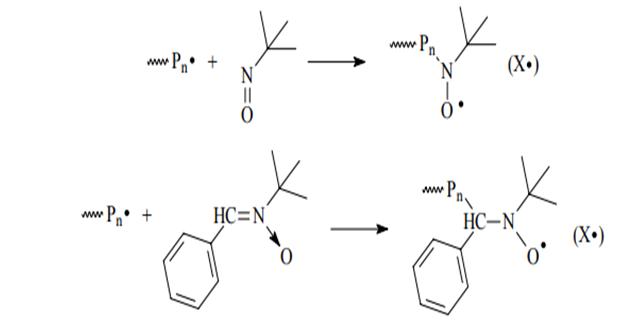
 Further, X • interacts with the growth radical (~ Pn•) with education labile communication C-ON <, (circuit 18). In the future, the process proceeds according to the conventional radical polymerization scheme according to the SFRP mechanism.
Further, X • interacts with the growth radical (~ Pn•) with education labile communication C-ON <, (circuit 18). In the future, the process proceeds according to the conventional radical polymerization scheme according to the SFRP mechanism.
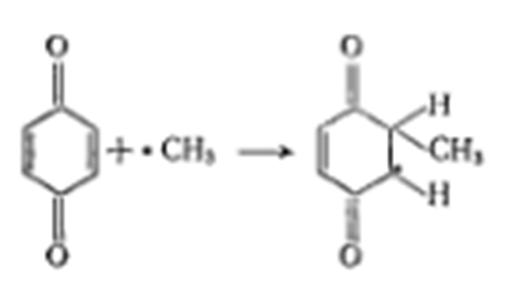
Draw the scheme of the mechanism of radical polymerization inhibition by quіnons.
The principle of polymer stabilization to destruction, proceeding along the radical mechanism, consists in the inhibition of the chain process (similar to the inhibition of chain polymerization). The general inhibition scheme can be expressed by the following equation

As shown in the present study, in the case of oxide-chromium catalysts, in the breakdown of polymerization with methanol labeled with C11, radioactivity appears in the polymer, and radioactivity is absent when the polymer is tritiated with alcohol by hydroxyl hydrogen. [32]
The peroxides formed by the addition of oxygen to vinyl chloride, together with sodium sulfite, chloride iron or sodium hydrosulfite, can be used as redox catalysts that initiate the polymerization of vinyl chloride, despite the inhibition of polymerization by free oxygen. Of these, the most active is sodium sulphite. Triethanolamine and dimethylaniline inhibit polymerization.
Constant of inhibation.

Дата добавления: 2018-02-15; просмотров: 610; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
