Use of nitroxide-mediated radical polymerization for controlled synthesis of gradient copolymers.
In the chain polymerization of macromolecular elements as a result of the disclosure of multiple bonds or cycles of monomers under the action of active centers located on the ends of growing chains on them.There are three types of active sites in chain polymerization - a radical, a cation and an anion, whereby radical, cationic and anionic polymerizations are distinguished.
Only compounds containing double carbon-carbon bonds are subjected to radical polymerization. In this case, the active center is carbradical, i.e. a carbon atom having one unpaired electron. Such an atom (radical) is very reactive due to the tendency of an unpaired electron to form a pair with a second electron. Compounds containing a π-bond are a suitable object for realizing this trend, since π-bond electrons are much more weakly bound than σ-bonds. Therefore, the radical easily selects one of the π-bond electrons in order to form a pair of electrons, that is, a new σ-bond:
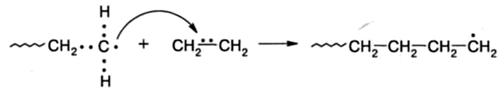
The chemical act described above is shown in the diagram for the polymerization of ethylene; the chemical bonds of the atoms participating in the reaction are represented electron pairs for clarity. The radical at the end of the growing chain is called the growth radical, and its reaction with the monomer - the main polymerization reaction - is a growth reaction. It can be seen from the scheme that the addition of the monomer to the growth radical is accompanied by the regeneration of the active site at the end of the chain - the carbon atom with the unpaired electr
Initiation of radical polymerizatio
The primary radicals necessary to initiate radical polymerization can be obtained by chemical reactions and by physical action on the monomer.
Real initiation. With the chemical or material interaction of substances that break up with the formation of free radicals, or a mixture of substances reacting with each other to form free radicals. As such methods, peroxides and azo compounds are commonly used, as well as combinations of substances forming the redox system.
Among the peroxides used are acyl-, alkyl-, hydroperoxides and peresters. The circle of azo compounds, practically limited as initiators, is more limited. The most famous among them is 2,2'-azobis (isobutyronitrile), which decomposes with the release of nitrogen:
Thanks to the latter circumstance, this and similar explanations in industry are not only initiators, but also for foaming plastics in the production of foams.
The initiators most used in modern research and production practice are given in Table. 5.1 with the characteristics of their decay. The table is closed by high-temperature initiators, which break up with the disruption of the C-C bond.
Oxidation-reduction systems are divided into two groups: organo- and water-soluble. To the first group of all the most common technologies, benzoyl peroxide is dimethylaniline. As a result of the oxidation-reduction reaction in this system, the primary act of which is the transfer of the electron from the amine to the peroxide, a benzo-at-radical is formed, which further realizes the polymeriz ation process:
Use of stable free radical polymerization for synthesis of low-molecular acrylates.

Дата добавления: 2018-02-15; просмотров: 694; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
