Initiation through strong anion
Cationic polymerization can be considered as another category of chain growth polymerization reactions. A cation initiates this reaction by transferring its charge to a monomer, which then results in producing a more reactive species. Next, the reactive monomer reacts similarly with other monomers to form a polymer. There are only a limited number of monomers which can facilitate the cationic polymerization chain reaction. Olefins containing electron-donating substituents and heterocycles are suitable for these types of reactions.

Initiation by protic acids
Discuss gel point in three-dimensional free-radical polymerization.
Gel formation theory applied to network formation processes proceeding according to the polycondensation mechanisms was groundlessly extended to network formation processes that develop according to the mechanisms of radical polymerization. The observed discrepancy between theoretically calculated values of critical conversion αcr (also called gel point) and experimentally measured values (αexp)cr was usually explained by secondary process of minor cycles formation (cyclization), the contribution of which is especially high in the case of three-dimensional free-radical polymerization (TFRP) for certain reasons. However, only in very recent times have researchers managed to implement such TFRP processes, to which the contribution of cyclization is negligibly small: these are TFRP in the living chains mode and copolymerization of network-forming (polyunsaturated) monomers, Mp, and with monounsaturated (non-network-forming) monomers Mm, with the proportion of components Mp to Mm exceeding a certain critical value (Mm/Mp)cr. The discrepancy between theoretical and experimental data observed in these processes could no longer be explained by cyclization; it became evident that these discrepancies represent the result of wrongly extending the Flory–Stockmayer approaches to the field of TFRP.
The value of critical conversion αcr in the case of formation of cross-linked polymers (the so-called gel point) characterizing the transition of the system from the fluid state to the state with an equilibrium elasticity modulus represents an important parameter of the system from both the technological and theoretical standpoints. Formation of an insoluble gel could lead to difficulties in technological processes for polymer production, and contrariwise, association of the critical conversion value with structural and kinetic parameters of the process determines the possibility of obtaining a cross-linked polymer with required physical and mechanical characteristics. From the theoretical standpoint, the gel point determines the percolation threshold in a polymer system being formed, and identification of the dependency of this value upon kinetic and structural characteristics is an important problem.
Calculations intended for finding αcr became especially important most recently,
Use of RAFT-polymerization for controlled synthesis of liquid crystal polymers.
In the past, majority of side chain liquid crystal materials were made by two synthetic routes – free radical polymerization of acrylic type monomers, bearing mesogenic moieties and hydrosilylation of mesogenic terminal alkenes with linear poly[(methylhydro)siloxanes], copolymers bearing alkylhydrosiloxane monomeric units or polymer systems modified with reactive Si-H bonds . These methods could be, in principle, applied only in synthesis of thermotropic SCLCP’s having different backbones, organic and rigid (acrylates) or purely inorganic, and flexible ones (polysiloxanes). The common feature of both types of SCLCPs, obtained by either of synthetic pathways, is high polydispersity index ( PDI = Mw/Mn >2 ). On a molecular level it means that there is a limited possibility of exact tuning the polymerization degree (length of individual macromolecules) and thus the liquid crystalline properties of the synthesized materials. Such the problems can be avoided once conditions for living or controlled polymerization are created or when liquid crystalline moieties are attached to star shaped or dendritic systems.
Living polymerizations including anionic, cationic and ring opening metathesis processes require in practice that, the rate constant of propagation is much higher than those of termination and chain transfer. Quantitative and fast initiation leads to polymers of controlled molecular weight and polydispersity, as well as allows for efficient synthesis of block copolymers. Such processes were widely studied in the past for syntheses of SCLCP and are still of some interest mainly in synthesis of SCLC poly(oxetanes). An interesting combination of cationic ring opening polymerization and “click” chemistry has been presented as a new promising synthetic route. BF3 catalyzed polymerization of 3-azidomethyl-3- methyloxetane led to a polymer backbone bearing side azide groups which then were coupled with propargyl monocholesterylsuccinate in the presence of CuI complex (Figure 1).
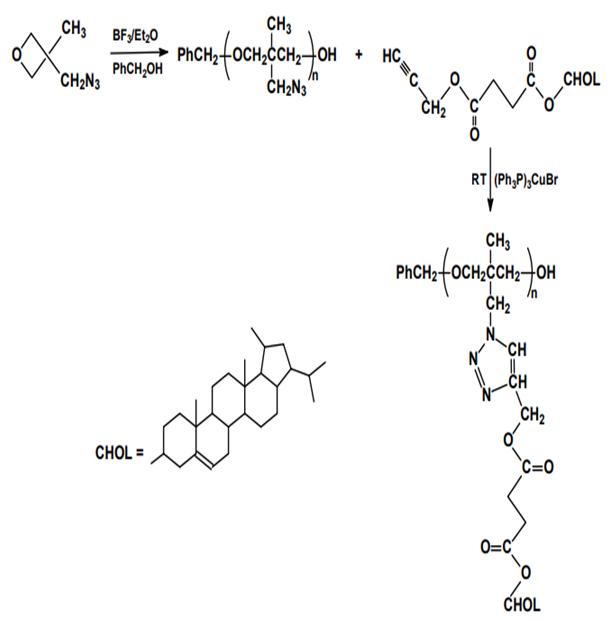
The current synthetic interest is, thus, focused on controlled radical polymerization – atom transfer radical polymerization (ATRP) and related reversible addition-fragmentation chain transfer technique (RAFT) . Controlled polymerizations have been applied to synthesize well defined homopolymers with controlled molecular weight, block copolymers, star polymers, etc.
Дата добавления: 2018-02-15; просмотров: 646; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
