Use of spin traps technique for kinetic investigation of elementary steps of RAFT-polymerization.
The mechanism of reversible addition-fragmentation chain transfer process as one of the widely used method of controlled living radical polymerization.




38. Propose method of determination of relative constants of chaintransfer tomonomer andinitiatorin free-radical polymerization.
An open circuit performs one of the following two mechanisms:
compounds (recombinations) of radicals 
disproportionation of radicals
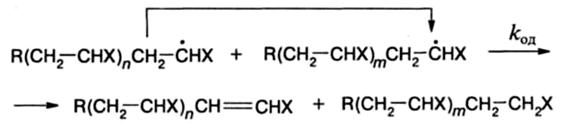
during which the hydrogen atom of the pre-terminal carbon of one radical is transferred to the terminal carbon atom of another radical. More often, the first reaction is realized, for example, in the polymerization of styrene and acrylates. The second reaction is typical for the polymerization of methyl methacrylate: the proportion of the growth radicals of this monomer reacting by disproportionation is 80% at 80 ° C. The breakaway mechanism can be determined by knowing the number of final groups-fragments of the initiator and the number of macromolecules. If the ratio of the first to the second is denoted by b, then λ. - the proportion of radicals participating in disproportionation is




At very high concentrations of the initiator, a breakdown on the primary radicals is possible, which leads to a decrease in the order of concentration of the initiator. In the limiting case, when all growing chains are terminated by primary radicals, equation

In case of heterogeneous polymerization, when the polymer precipitates, the coils of macroradicals are tightly folded, and the active end of the radical can be at the center of such a coil and / or inside the aggregate of adhering macromolecules not accessible to other radicals. This means the kinetic death of the growth radical, i.e. monomolecular breakage.
As a result of this reaction, the growth radical breaks off the mobile atom or group of atoms from the inactive molecule and deactivates, and the inactive molecule becomes the radical that gives rise to a new kinetic chain:

The reaction of chain transfer to a monomer in the polymerization of allylic monomers has a fundamentally different effect. The transmission constant on them is abnormally large and corresponds to 4 to 10 acts of normal connection for one act of transmission of the circuit. This is explained by the high mobility of allyl hydrogen, which in turn is due to the stabilization of the allyl radical formed:

Study of the mechanism of alternating copolymerization by spin traps method.
The most commonly used spin traps are alpha-phenyl N-tertiary-butyl nitrone (PBN) and 5,5-dimethyl-pyrroline N-oxide (DMPO). More rarely, C-nitroso spin traps such as 3,5-dibromo-4-nitrosobenzenesulfonic acid (DBNBS) can be used: often additional hyperfine information is derived, but at a cost of specificity (due to facile non-radical addition of many compounds to C-nitroso species, and subsequent oxidation of the resulting hydroxylamine).
5-Diisopropoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DIPPMPO) spin trapping has been used in measuring superoxide production in mitochondria.
A comprehensive list of Spin Trapping molecules is maintained by the IUPAC
A common method for spin-trapping involves the addition of radical to a nitrone spin trap resulting in the formation of a spin adduct, a nitroxide-based persistent radical, that can be detected using EPR. The spin adduct usually yields a distinctive EPR spectrum characteristic of a particular free radical that is trapped. The identity of the radical can be inferred based on the EPR spectral profile of their respective spin adducts such as the g value, but most importantly, the hyperfine-coupling constants of relevant nuclei. Unambiguous assignments of the identity of the trapped radical can often be made by using stable isotope substitution of the radicals parent compound, so that further hyperfine couplings are introduced or altered. It is worth noting that the radical adduct (or products such as the hydroxylamine) can often be stable enough to allow non-EPR detection techniques. The groups of London, and Berliner & Khrahmtsov have used NMR to study such adducts and Timmins and co-workers used charge changes upon DBNBS trapping to isolate protein adducts for study. A major advance has been the development of anti-DMPO antibodies by Mason's group, allowing study of spin trapping reactions by a simple immuno-based techniques.
Дата добавления: 2018-02-15; просмотров: 634; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
