A spontaneous thermodynamic destruction metal as a result of exposure to chemical and electrochemical environment
Задание #133
Вопрос : The process of redox decomposition of an ionic compounds by passing electricity through molten or aqueous solutions of ionic compounds is called:
Electrolysis
2) oxidation
3) dissociation
4) hydrolysis
5) corrosion
Задание #134
Вопрос : With which metal does oxygen combine to form rust?
1) Copper
2) Silver
3) Platinum
Iron
5) Gold
Задание #135
Вопрос : Which is the strongest acid in the following?
1) HClO3
2) H2SO4
HCl
4) HClO4
5) H2SO3
Задание #136
Вопрос : How long will it take 1.0 kg of gold (Au) to late from a solution of Au3+ with a current of 150 A?
1) 1 hours 35 minutes
2) 54.4 minutes
3) 2 days
4) 136 hours
Hours 43 minutes
Задание #137
Вопрос : Select a typical oxidizing agent:
1) KMnO4
2) СО
3) С
4) iron (II) compounds 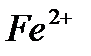
5) Н2
Задание #138
Вопрос : The reaction of the salt with water, whereby the salt is dissociated and decomposed to form a weak electrolyte (weak acid or weak base) is called ...
1) Electrolysis
2) Dissociation
3) Osmosis
Hydrolysis
5) Corrosion
Задание #139
Вопрос : In which of the following compounds, nitrogen exhibits highest oxidation state?
1) N2O 2) NH3 3) HNO2 4) N2 5) HNO3
Задание #140
Вопрос : In the common dry cell, the zinc atoms are:
1) precipitates at the cathode
2) oxidized at the cathode
Oxidized at the anode
4) reduced at the cathode
5) reduced at the anode
Задание #141
Вопрос : Which among the following is a set of transition elements?
1) Pt, Cd, As
Zr, Au, Ni
3) Sn, Bi, Mg
4) K, Mg, Fe
5) Hg, Pb, Ca
Задание #142
Вопрос : Select the oxidation process:
1) 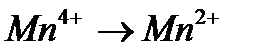
2) 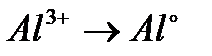
3) 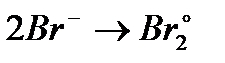
4) 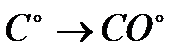
5) 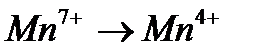
Задание #143
Вопрос : Which of the following metal ions will be discharge in first at electrode?
1) Al3+ (E° = -1,66V)
2) Cu2+ (E° = +0,34V)
3) H+ (E° = 0V)
4) Ag+ (E° = +0,80V)
5) Fe2+ (E° = -0,44V)
Задание #144
Вопрос : What kind of water is obtained by passing the natural water through a system of cation and anion exchangers:
1) mineral water
|
|
|
2) hardness
3) soft water
4) distilled water
5) moderately hardness water
Задание #145
Вопрос : An equivalent weight of oxidizer KMnO4 in a strong acidic medium is equal ... (g/mol):
1) 52,6
2) 158
3) 25,02
4) 79
5) 31,6
Задание #146
Вопрос : Which metal is extracted from Bauxite?
1) Gold
2) Aluminum
3) Tungsten
4) Arsenic
5) Iron
Задание #147
Вопрос :In an electrolytic cell:
1) potential energy decreases
2) kinetic energy decreases
3) potential energy changes into electrical energy
4) electrical energy changes into chemical energy
5) chemical energy changes into electrical energy
Задание #148
Вопрос : An equivalent weight of oxidizer KMnO4 in a basic medium is equal ... (g/mol):
1) 79 2) 25,02 3) 158 4) 31,6 5) 52,6
Задание #149
Вопрос : Brass is ...
1) A Compound
2) An Alloy
3) A Mixture
4) A Substance
5) An Element
Задание #150
Вопрос : ………………. among the following contain partially filled d - subshell and does not show variable oxidation states.
1) Cd
2) La
3) Hg
4) Cr
5) Mn
Задание #151
Вопрос : Medium reactivity metals are obtained from their oxides by ...
1) Roasting
2) Reduction
3) Heating
4) Electrolytic Separation
5) Oxidation
Задание #152
Вопрос : Which among the following is not true for electrochemical cell?
1) It needs a porous partition
2) It consists of generally two electrolytes
3) Anode acquires negative charge
4) Cathode acquires positive charge
It consists of a battery
Задание #153
Вопрос : Which metals acts as a protector for the iron and steel constructions?
1) Zn, Mg 2) Sn, Pb 3) Cd, Na 4) Cu, Mg 5) Ni, Zn
Задание #154
Вопрос : The process of reduction of ores involving heating and melting with carbon is known as:
1) Thermite process
2) Roasting
|
|
|
3) Pyrolysis
4) Smelting
5) Zone refining
Задание #155
Вопрос : Calculate the electrode potential at an gold electrode, dipped in a 0,001М aqueous solution of gold salt at 25°C 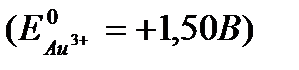 :
:
1) +1,481V 2) -1,45V 3) +1,441V 4) +1,557V 5) +1,559V
Задание #156
Вопрос : German silver is an alloy of zinc and:
1) Fe and Ni
2) Cu and Ni
3) Sn and Ni
4) Al and Fe
5) Cu and Fe
Задание #157
Вопрос : Which of the following metal ions, as Al3+, Na+, Mg2+, Ag+, will be discharged in first at electrode during molten electrolysis?
1) Na+ , Ag+ , Al3+ , Mg2+
2) Al3+, Na+, Mg2+, Ag+
3) Ag+, Al3+, Mg2+, Na+,
4) Ag+, Na+, Mg2+, Al3+
5) Mg2+, Al3+, Na+, Ag+
Задание #158
Вопрос : Hot concentrated sulfuric acid (H2SO4) acts as moderately strong oxidising agent. It oxidises both metals and nometals. Which of the following element is oxidised by conc. H2SO4 into gaseous products?
1) Cu 2) Zn 3) S 4) NaCl 5) C
Задание #159
Вопрос : The chemical equivalent of the metal is 19.3. When 3A of electric current is passed for 50 min. What will be the amount of metal deposited from an electrolyte?
1) 3.6 g 2) 0.9 g 3) 4.2 g 4) 0.03 g 5) 1.8 g
Задание #160
Вопрос : In the electrochemical series metals are arranged ...
1) in the order of decreasing the electrode potential
2) depending on the resistance to oxidation by oxygen
3) depending on the resistance to oxidation by strong acids
4) in the order of weakening metal solubility in water
5) in the order of increasing the electrode potential
Задание #161
Вопрос : What is the oxidation state of the central metal atom in the complex [Cu(NH3)4]SO4 :
1) 0 2) +4 3) +3 4) +2 5) -2
Задание #162
Вопрос : What will be the electrode potential at an aluminium electrode, dipped in a 0,001М AlCl3 aqueous solution at 25°C 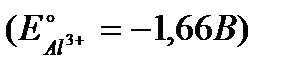 :
:
|
|
|
1) -1,601V 2) -1,84V 3) -1,719V 4) -1,54V 5) -1,64V
Задание #163
Вопрос : Which metal will serve as the cathode for iron?
1) Cu, Ni 2) Zn, Mg 3) Zn, Al 4) Cd, Mg 5) Ni, Zn
Задание #164
Вопрос : Often to prevent corrosion, metals are galvanized by covering them with a layer of ...
1) Lead 2) Copper 3) Zinc 4) Tin 5) Sodium
Задание #165
Вопрос : What is the oxidation state (OS) of the central metal atom and the coordination number (CN) in the complex Na3[Co(H2O)2 Cl2 PO4] :
1) OS = -2; CN = 5
2) OS = +3; CN = 9
3) OS = +1; CN = 4
4) OS = +5; CN = 9
5) OS = +2; CN = 5
Задание #166
Вопрос : Water hardness is characterized by the presence of calcium and magnesium ions, called ...
1) total
2) temparary hardness
3) permanent hardness
Carbonate
5) technical
Задание #167
Вопрос : What is the sign of ΔG for a galvanic cell?
1) 1
Negative
3) Zero
4) Either negative or positive
5) Positive
Задание #168
Вопрос : Bronze is an alloy of ...
1) Zink, Iron and Carbon
Copper and Tin
3) Barium, Zinc and Iron
4) Copper and Zinc
5) Lead and Copper
Задание #169
Вопрос : This type, of corrosion occurs due to direct chemical attack of environment or atmospheric gases like oxygen halogens, hydrogen sulphide, sulphurdioxide, nitrogen or anhydrous inorganic liquid with metal surfaces in immediate proximity. What is the corrosion type?
Cavitation corrosion
2) galvanic corrosion
3) chemical corrosion
4) gas corrosion
5) liquid corrosion
Задание #170
Вопрос : Of four different laboratory solutions, the solution with the highest acidity has a pH of:
1) 2 2) 9 3) 11 4) 3 5) 5
|
|
|
Задание #171
Вопрос : What is the equivalent weight of KMnO4 when it is converted in MnO2?
1) M/1 2) M/3 3) M/5 4) M/4 5) M/2
Задание #172
Вопрос : Mercury is the only metal which is liquid at 0°C because it has:
1) High vapour pressure
Дата добавления: 2018-09-20; просмотров: 243; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
