The positive charged electrode at which reduction occurs
Задание #33
Вопрос : The cyanide complex used for the extraction of Gold is:
1) [Au (CN)] -
2) [Au (CN) 6] -
3) [Au (CN) 2] -
4) [Au (CN) 3] -
5) [Au(CN) 4] -
Задание #34
Вопрос : Helium is used in balloons and airships in place of hydrogen because it is ...
1) more abundant than hydrogen
2) lighter than hydrogen
3) flammable
Incombustible
5) radioactive
Задание #35
Вопрос : The coordination number of the complex compound Ni(NH3)6(NO3)2 is equal ...
1) 4
2) 6
3) 8
4) 5
5) 2
Задание #36
Вопрос : With decrease in size of cation:
1) Complex forming tendency does not depend to size of cation
2) None of these
3) Complex forming tendency increases
4) Complex forming tendency does not change
5) Complex forming tendency decreases
Задание #37
Вопрос : Four alkali metals K, L, M, N and P are having standard electrode potentials as -4.05, -2.66, -0.50, 0.70 V and 0.25V respectively. Which one of the following is most reducing agent?
1) P
K
3) L
4) N
5) M
Задание #38
Вопрос : What the metal cations will be recovered from their salt solutions by magnesium?
1) Li, Sn
2) Cu, K
3) Ca, Na
4) Ag, Ca
5) Zn, Mn
Задание #39
Вопрос : What is the total hardness of water (mmol/L), if its 1 liter contains 40,04 mg of calcium (Ca2+) ions and 48.24 mg magnusium (Mg2+) ions?
1) 2 2) 4 3) 5 4) 6 5) 3
Задание #40
Вопрос : Cathode is:
1) the neutral charged electrode at which reduction occurs
2) the positive charged electrode at which reduction occurs
3) the negative charged electrode at which oxidation occurs
4) the electrode at which oxidation occurs
5) the electrode at which reduction occurs
Задание #41
Вопрос : Which salt is hydrolyzed by anion?
1) BaCl2
2) FeCl3
3) AgNO3
4) CH3COONa
5) Pb(NO3)2
Задание #42
Вопрос : For the following reduction reaction how many coulombs are required? 1 mol of Cu2+ to Cu:
1) 96500 C
2) 482500 C
3) 386000 C
4) 9650 C
C
Задание #43
Вопрос : Coinage metals show the properties of:
|
|
|
1) Normal elements
2) Alkali metals
3) Typical elements
4) Transitional elements
5) Inert elements
Задание #44
Вопрос : Select cause of electrochemical corrosion:
1) Any two metals with different potentials in contact, dipped in an electrolyte.
2) Metal react with water
3) Metal react with non-electrolyte liquid
4) Metal react with acid
5) Metal react with alkili
Задание #45
Вопрос : During electrochemical corrosion of lead pipes in acidic soil, here takes place the following chemical process:
1) Pb2+ +2e→Pb , 2H+ +2e→ H2
2) 2Pb-4e→2Pb2+, 4OH- -4e→2H2O +O2
3) Pb-2e→Pb2+ , 2H+ +2e→H2
4) 2Pb-4e→2Pb2+ , 2H2O+O2 +4e→4OH-
5) Pb-2e→Pb2+ , Pb2+ +2e→Pb
Задание #46
Вопрос : Weak basis and weak acid that form salt XA, if such ionic - molecular equation X+ + A- + H2O = XOH + HA correspond to its hydroysis. What salt is it?
1) BaCl2
2) CrCl3
3) AlCl3
4) NaNO3
5) NH4NO2
Задание #47
Вопрос : Which one of the following orders correctly represents the increasing acid strengths of the given acids?
1) HOClO2 < HOClO3 < HOClO < HOCl
2) HOCl < HOClO < HOClO2 < HOClO3
3) HOClO < HOCl < HOClO3 < HOClO2
4) HOClO3 < HOClO2 < HOClO < HOCl
5) HOClO3 < HOClO < HOCl < HOClO2
Задание #48
Вопрос : What is the equivalent weight of KMnO4 when it is converted in К2MnO4?
1) M/4
2) M/5
3) M/2
4) M/3
5) M/1
Задание #49
Вопрос : In an electrochemical galvanic cell:
1) potential energy decreases
2) potential energy changes into electrical energy
Chemical energy changes into electrical energy
4) kinetic energy decreases
5) electrical energy changes into chemical energy
Задание #50
Вопрос : What salt is not hydrolyzed?
1) Na2CO3
2) Pb(NO3)2
3) BaCl2
4) K3PO4
5) K2S
Задание #51
Вопрос : Which of the following metals is often found in pure state?
|
|
|
1) Copper
2) Magnesium
3) Iron
Gold
5) Aluminum
Задание #52
Вопрос : What is the sign of EMF for a galvanic cell?
1) Zero
Positive
3) 1
4) Either positive or negative
5) Negative
Задание #53
Вопрос : Elements found in D-block of periodic table are known as ...
1) alkali metals
Transition elements
3) earth alkali metals
4) noble elements
5) metaloids
Задание #54
Вопрос : Which metal is common between brass, bronze and German silver?
1) Mg
2) Ag
Zn
4) Cu
5) Al
Задание #55
Вопрос : Where does the acid base indicator phenolphthalein gain of crimson (purple) colour?
1) Na2 С O3
2) SnCl2
3) MgSO4
4) KI
5) AgNO3
Задание #56
Вопрос : A potassium permanganate KMnO4 is a strong oxidizing agent in ................ medium
Acidic
2) in water
3) neutral
4) in ammonium
5) basic
Задание #57
Вопрос : The Nernst equation is described by:
1) 
2) 
3) 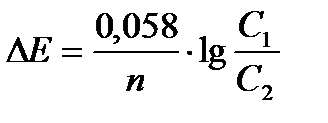
4) 
5) 
Задание #58
Вопрос : Corrosion of iron is essentially an electrochemical phenomenon where the cell reaction are ...
1) Fe is oxidised to Fe2+ and H2O is reduced to O2
2) Fe is oxidised to Fe3+ and H2O is reduced to O2-
3) Fe is oxidised to Fe2+ and dissolved oxygen in water is reduced to OH-
4) Fe is oxidised to Fe2+ and H2O is reduced to O2-
5) Fe2+ is oxidised to Fe3+ and H2O is reduced to H2
Задание #59
Вопрос : On heating lead nitrate form oxides of nitrogen and lead. The oxides formed are ...
1) NO and PbO
2) NO2 and PbO
3) NO2 and PbO2
4) N2O and PbO2
5) NO and PbO2
Задание #60
Вопрос : An intramolecular reaction in which the atoms of one element is reduced and simultaneously increase the degree of oxidation state is called:
1) Displacement
2) Disproportionation
3) Combustion
4) Decomposition
Intermolecular
Задание #61
|
|
|
Вопрос : This type of corrosion occurs when the metal comes in contact with a conducting liquid or when two dissimilar metals are immersed or dipped partly in a solution. There is the formation of a galvanic cell on the surface of metals. Some parts of the metal surface act as anode and rest act as cathode. Water must be present to serve as a medium for the transport of ions. What is the corrosion type?
Electrochemical corrosion
2) liquid corrosion
3) gas corrosion
4) atmospheric corrosion
5) chemical corrosion
Задание #62
Вопрос : Select the reduction process:
1) 
2) 
3) 
4) 
5) 
Задание #63
Вопрос : Why is it necessary to add some salts to water for the electrolysis of water to proceed?
1) Water are electrolyzed spontaneously
2) Salts are necessary to prevent the explosion of the dangerous gasses hydrogen and oxygen
3) Salts are not needed for the reaction to proceed
4) Due to the production of acid during the electrolysis, a buffer is needed to maintain a constant pH
Дата добавления: 2018-09-20; просмотров: 389; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
