It has high negative reduction potential
4) it does not corrode easily
5) it is an alkali metal
Задание #98
Вопрос : Give the line notation for a cell that reduces PbSO4 to Pb and oxidizes Fe to Fe2+.
1) Fe (s) | Pb2+ (aq) || Fe2+ (aq) | Pb (s)
2) Pb (s) | Pb2+ (aq), SO4 2- (aq) || Fe2+ (aq), SO4 2- (aq) | Fe (s)
3) Fe (s) | Fe2+ (aq), SO4 2- (aq) || Pb2 (aq), SO4 2- (aq) | Pb (s)
4) Pb (s) | Pb2+ (aq) || Fe2+ (aq) | Zn (s)
5) Fe (s) | Fe2+ (aq) || Pb2+ (aq) | Pb (s)
Задание #99
Вопрос : For 0,01М sodium hydroxide aqueous solution (NaOH) its рH value will be equal:
1) 4 2) 8 3) 12 4) 10 5) 2
Задание #100
Вопрос : Roasting of sulphides gives the gas X as a byproduct This is a colorless gas with choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic acts as a reducing agent and its acid has never been isolated. The gas X is ...
1) SO2 2) SO3 3) S 4) CO2 5) H2S
Задание #101
Вопрос : The most dangerous type of corrosion to the national economy is ...
1) chemical corrosion
2) liquid corrosion
Atmospheric corrosion
4) gas corrosion
5) galvanic corrosion
Задание #102
Вопрос : Which of the following statements is not correct for the following reaction?
Ag + CuSO4 → Ag2SO4 + Cu
1) Ag is less reactive than Cu
2) Cu is more reactive than Ag
3) Ag is displaced Cu2+ ion in its salt solution
4) The blue colour solution is CuSO4
5) The reaction does not occur
Задание #103
Вопрос : For an aqueous solution with pH=10,  hydroxide ions concentration will be equal to.... (mol/L):
hydroxide ions concentration will be equal to.... (mol/L):
1) 10-4 2) 10-5 3) 10-2 4) 10-10 5) 10-9
Задание #104
Вопрос : Which noble gas is most abundant in atmosphere?
1) He 2) Ar 3) Ne 4) Xe 5) Kr
Задание #105
Вопрос : Indicate the oxidation state central metal atom and coordination number of complex ion [FeF6]3−
1) Oxidation state of Fe = +3; Coordination number = 6
2) Oxidation state of Fe = -3; Coordination number = 6
3) Oxidation state of Fe = +6; Coordination number = 6
4) Oxidation state of Fe = +2; Coordination number = 6
|
|
|
5) Oxidation state of Fe = +4; Coordination number = 6
Задание #106
Вопрос : What is the total hardness of water, if for titration of 50 ml of water was consumed 2.5 ml of 0.025 N solution of EDTA (mmol / L)?
1) 1,25 2) 2,20 3) 2,75 4) 2,22 5) 3,45
Задание #107
Вопрос : What is the oxidation state of the central metal atom in the complex Na2[Co(NH3)2Cl4] :
1) +3 2) +1 3) 0 4) +4 5) +2
Задание #108
Вопрос : From a mixture of cations in the solution are recovered in the first place:
1) Discharged anions with the lowest value of the reducing potential
2) Water is discharged
3) Discharged anions with the greatest value of the reducing potential
Metals with the lowest value of the electrode potential
5) Metals with the greatest value of the electrode potential
Задание #109
Вопрос : What the metal cations will be recovered from their salt solutions by iron?
1) Zn, Mn
2) Ca, Cu
Sn, Pb
4) Mn, Ni
5) Al, Cu
Задание #110
Вопрос : What is a Faraday?
1) a unit of current
A unit of charge
3) a unit of energy
4) a mole of electrons
5) a mole of electrolyte
Задание #111
Вопрос : Among the following which is not a true statement for Faraday’s laws of electrolysis?
1) The volume of gases reduced or oxidized is directly proportional to the quantity of electricity passed
2) The weight of substance deposited is directly proportional to the quantity of electricity passed
3) The weight of substance deposited at the respective electrodes are directly proportional to their equivalent weights
4) The weight of substance deposited is directly proportional to the quantity of electricity passed
The weight of substance deposited is not directly proportional to the quantity of electricity passed
|
|
|
Задание #112
Вопрос : Hydrogen gas is not liberated when the following metal is added to dil. HCl ...
1) Zn 2) Ag 3) Sn 4) Mg 5) Fe
Задание #113
Вопрос : Select the oxidation process:
1) 
2) 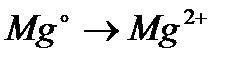
3) 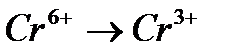
4) 
5) 
Задание #114
Вопрос : Which among the following is true about transition metals?
1) All of these
2) Form complexes
3) None of these
4) Paramagnetic
5) Shows variable oxidation state
Задание #115
Вопрос : Ca and Cu metals when coupled together gives maximum EMF for a voltaic cell because:
1) Ca show negative electrode potential and Cu show positive electrode potential
2) It shows same standard reduction potential value
3) It shows less difference in standard reduction potential value
Дата добавления: 2018-09-20; просмотров: 320; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
