Both galvanic cell and voltaic cell
THE MINISTRY OF AGRICULTURE THE REPUBLIC OF KAZAKHSTAN
S. SEIFULLIN KAZAKH AGROTECHNICAL UNIVERSITY
DEPARTMENT OF PHYSICS AND CHEMISTRY
Author: Mukhanbetova N. A, senior teacher,
master of chemistry
TEST for 2nd control
On the discipline “GENERAL and INORGANIC CHEMISTRY”
for students of technical specialties:
5B072700 – Technology of food products
and
5B080600 – Agrarian technique and technology
Test for 2nd control was discussed at the meeting of the chair chemistry and physics "_____" ___________ 2017, protocol № _______
Head of the Department ________________Alimkulova E.J., associate prof.
Astana 2017
Задание #1
Вопрос : Which metals will serve as the anode for lead?
1) Ag, Fe
2) Co, Pt
3) Pb, Cu
Cu, Al
5) Cr, Ni
Задание #2
Вопрос : Use the activity series given to you to determine which of the following reactions will NOT take place:
1) Cu + HCl -->
2) Mg + H2O -->
3) Ag + AuCl3 -->
4) Zn + HNO3 -->
5) Ca + Pb(NO3)2 -->
Задание #3
Вопрос : What metals will not react with diluted acids?
Pt, Au
2) Ni, Mg
3) Zn, Fe
4) Au, Cа
5) Cd, Cu
Задание #4
Вопрос : If iron filings are put in different test tubes containing ZnSO4, CuSO4, Al2(SO4)3, CaCl2, CrCl3 in which one changes will be observed?
1) CuSO 4
2) CaCl2
3) Al2(SO4)3
4) ZnSO4
5) CrCl3
Задание #5
Вопрос : A current of x A flowing for 10 min deposits 3g of the metal which is monovalent. The atomic mass of the metal is 50. Find the value of x?
1) 0.10
2) 9.65
3) 6.72
4) 8.75
5) 10.5
Задание #6
Вопрос : The Earth's atmosphere or air is composed of several gases. By far, the most abundant gas in the Earth's atmosphere is ...
Nitrogen
2) Hydrogen
3) Carbon dioxide
4) Argon
5) Oxygen
Задание #7
Вопрос : The electrode potential at an copper electrode, dipped in a 0,01М aqueous solution of copper sulfate at 25°C, is equal ....... 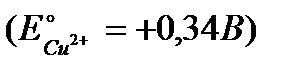 :
:
|
|
|
1) +0,34В
2) +0,369В
3) +0,281В
4) +0,399В
5) +0,311В
Задание #8
Вопрос : What is the pOH of the solution, with a content of [H+] = 10-4 mol /L?
1) 10
2) 8
3) 4
4) 6
5) 9
Задание #9
Вопрос : Galvanization of iron sheets is done by:
1) Pb plating
2) Tin plating
3) Ag plating
Zn plating
5) Cu plating
Задание #10
Вопрос : Select the definition of Faraday's 1st law:
1) A chemical compound always contains exactly the same proportion of elements by mass.
2) For non-electrolyte solutions, the partial vapor pressure of a solvent over a solution (P1) is equal to the vapor pressure of the pure solvent (P0) multiplied by the mole fraction of the solute (X2).
3) At constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of reacting species, with each concentration term raised to the power equal to the numerical coefficient of the species in the chemical equilibrium
The mass of a substance produced at an electrode during electrolysis is proportional to the number of moles of electrons (the quantity of electricity) transferred at that electrode
5) The mass of a substance deposited or liberated at any electrode on passing a certain amount of charge is directly proportional to its chemical equivalent weight
Задание #11
Вопрос : In a comparison with S-block elements, melting point of transition elements are ...
Higher
2) does not matter because they are same
3) same
4) lower
5) constant
Задание #12
Вопрос : In a redox reaction if the EMF is positive then the reaction is:
1) Reversible
2) Non-spontaneous
3) None of these
Spontaneous
5) Irreversible
Задание #13
Вопрос : All alloys contain this element if they are amalgama. The element is:
1) platinum
2) gold
Mercury
4) iron
5) zinс
Задание #14
|
|
|
Вопрос : Coinage metals show the properties of:
1) Active elements
2) Typical elements
Transitional elements
4) Inert elements
5) Normal elements
Задание #15
Вопрос : Use the activity series given to you to determine which of the following reactions will take place:
1) Hg + H2O =>
2) NaCl + Mg =>
3) Zn + Al2O3 =>
4) Ag + HNO3 (dil) =>
5) CuO + H2 =>
Задание #16
Вопрос : X ampere current is passed for y seconds through copper and silver voltameters. The metal that is deposited more is:
1) both is deposited equally
2) Cu
Ag
4) Al
5) none of these
Задание #17
Вопрос : Calculate the standard cell potential if the standard reduction potential of Cu and Zn are + 0.34V and -0.76V respectively. The cell is shown below Zn / ZnSO4 // CuSO4 / Cu.
V
2) 0.42V
3) -0.42V
4) 1.50V
5) 4.30V
Задание #18
Вопрос : Write down the IUPAC name of the following complex [Co(NH3)5Cl]Cl2 and indicate the oxidation state and coordination number.
1) Pentaamminechloridocobalt (III) chloride. Oxidation state of Co = +3; Coordination number = 8
2) Pentaamminechloridocobalt (II) dichloride. Oxidation state of Co = +2; Coordination number = 6
3) Pentaamminechloridocobalt (III) chloride. Oxidation state of Co = +3; Coordination number = 5
4) Pentaamminechloridocobalt (III) dichloride. Oxidation state of Co = +3; Coordination number = 6
5) Pentaamminechloridocobalt (III) monochloride. Oxidation state of Co = +3; Coordination number = 8
Задание #19
Вопрос : Rocks rich in metals are known as ...
1) Ores
2) Allotropes
3) Slag
Metalloids
5) Gangue
Задание #20
Вопрос : Why do most ships with iron hulls hang from those hulls a piece of magnesium?
1) The magnesium has a greater reduction potential than the iron and therefore acts as an anode, being dissolved into the ocean, while the ship's hull acts as a cathode and is thus protected against corrosion.
2) The magnesium has a greater reduction potential than the iron and therefore acts as a cathode, being dissolved into the ocean, while the ship's hull acts as an anode and is thus protected against corrosion.
|
|
|
3) none of these
4) The magnesium has a greater oxidation potential than the iron and therefore acts as an anode, being dissolved into the ocean, while the ship's hull acts as a cathode and is this protected against corrosion.
The magnesium has a greater oxidation potential than the iron and therefore acts as a cathode, being dissolved into the ocean, while the ship's hull acts as an anode and is thus protected against corrosion.
Задание #21
Вопрос : Select the correct scheme of the zinc-copper galvanic cell:
1) (-) Zn//Cu(+)
2) (+)Cu/CuSO4// ZnSO4/Zn(-)
3) (+)Cu/ZnSO4//CuSO4/Zn(-)
4) (-)ZnSO4/Zn//Cu/ CuSO4(+)
5) (-)Zn/ZnSO4//CuSO4/Cu(+)
Задание #22
Вопрос : Compounds in which a central metal atom or ion is linked to a number of ions or neutral molecules by coordinate bonds or which contain complex ions is called:
1) coordination compounds
2) double salt
3) ionic compounds
4) salt
Ligands
Задание #23
Вопрос : Ductility is the ability for a metal to be
1) melted into molds
2) pressed into angles
3) rolled into sheets
Pulled into wires
5) reflected lifgt from it surface
Задание #24
Вопрос : For the following reaction  . Calculate the standard EMF of the cell. Given
. Calculate the standard EMF of the cell. Given 
1) +0.80 volt
2) -0.76 volt
3) -1.56 volt
4) +1.56 volt
5) +0.04 volt
Задание #25
Вопрос : A device in which electric current is produced at the expense of spontaneous chemical reaction:
1) voltaic cell
2) voltameter
3) electrolytic cell
both galvanic cell and voltaic cell
5) galvanic cell
Задание #26
Вопрос : Select the reduction process:
1) 
2) 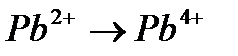
3) 
4) 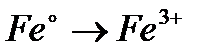
5) 
Задание #27
Вопрос : I will be describing a chemical element. Try to identify it with the fewest number of clues. This chemical element is a silver-white metal, with bluish tinge, capableof taking a high polish. It is element occurs bundantly in all ordinary rocks, except limestone and sandstone; is third in abundance of the elements in the earth's crust and used in aviation and to make drink cans.The atomic number of this element is 13. Name it element:
|
|
|
1) Cr
Al
3) Zn
4) Os
5) Pb
Задание #28
Вопрос : In which of the following compounds does O have an oxidation state not equal to -2?
1) H2O
2) H2SO4
3) ClO4 -
4) NaOH
HOF
Задание #29
Вопрос : Sodium nitroprusside, Na2 [Fe (CN)5 (NO)], is an octahedral complex given to heart transplant patients. It contains the nitrosonium ligand, NO+, which is one of the few known cationic ligands. Given this, determine the oxidation state of Fe in sodium nitroprusside.
1) 0
2) +3
3) +2
4) - 1
5) + 1
Задание #30
Вопрос : Galvanization of iron sheets is done by:
1) Tin plating
2) Cu plating
3) Pb plating
4) Ag plating
Zn plating
Задание #31
Вопрос : What is the electrode potential of a half cell consisting of zinc electrode in 0,01М ZnCl2 aqueous solution at 25°C  :
:
1) -0,789В
В
3) -0,819В
4) -0,76В
5) -0,731В
Задание #32
Вопрос : Anode is:
1) the electrode at which reduction occurs
2) the neutral charged electrode at which oxidation occurs
3) the negative charged electrode at which oxidation occurs
4) the electrode at which oxidation occurs
Дата добавления: 2018-09-20; просмотров: 460; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
