It shows greater difference in standard reduction potential values
5) None of these
Задание #116
Вопрос : Use the activity series given to you to determine which of the following reactions will NOT take place:
1) Zn + NiCl2 =>
2) Fe + CuSO4 =>
3) K + H2O =>
4) Al + Cr2O3 =>
5) Au + HNO3 (conc) = >
Задание #117
Вопрос : An aqueous solution of K2SO4 is electrolysed using platinum electrodes. The products at the anode and the cathode are:
1) SO4-2, H2
2) O2, H2
3) KOH, H2SO4
4) O2, K
5) SO42-, К
Задание #118
Вопрос : What are metals that can be beaten into various shapes called?
1) Beatable Metals
2) Polymer Metals
3) Malleable Metals
4) Shiny Metals
5) Ductile Metals
Задание #119
Вопрос : Determine the standard EMF of the cell 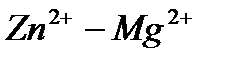 :
: 
1) +1,60В
2) +3,12В
3) -1,60В
4) -3,12В
5) -2,36В
Задание #120
Вопрос : Iron nails were dipped in a solution kept in a test tube. After half an hour, it was observed that the colour of the solution had changed. Which solution was in the test tube?
1) Na2SO4
2) Al2(SO4)3
3) MgSO4
4) ZnSO4
5) CuSO4
Задание #121
Вопрос : Which of the following reactions involve NEITHER oxidation nor reduction?
1) reaction of potassium hydroxide with nitric acid
2) displacement reaction of iron in copper sulfate
3) burning antimony in chlorine
4) decomposition of potassium chlorate
5) reaction of sodium with water
Задание #122
Вопрос : What are metals that can be stretched easily known as?
1) Polymer Metals
2) Shiny Metals
3) Malleable Metals
4) Ductile Metals
5) Stretchable Metals
Задание #123
Вопрос : What is the oxidation state (OS) of the central metal atom and the coordination number (CN) in the complex Co(NH3)5 Cl Cl ? Name it
1) OS = +3, CN = 7. Pentaaminodichloridocobalt (III)
2) OS = +3, CN = 6. Pentaaminochloridocobalt (III) chloride
3) OS = +2, CN = 16. Pentaaminochloridocobaltat (II) chloride
4) OS = +2, CN = 6. Pentaaminochloridocobalt (II) chloride
5) OS = +1, CN = 16. Pentaaminochloridocobalt (I) chloride
6) OS = +2, CN = 5. Pentaaminochloridocobaltat (II) chloride
Задание #124
Вопрос : Which metal is common between brass, bronze and German silver?
|
|
|
1) Ti 2) Mg 3) Cu 4) Al 5) Zn
Задание #125
Вопрос : Determine the standard EMF of the cell  :
:

1) +0,74V 2) +0,55V 3) +0,19V 4) +0,93V 5) -0,93V
Задание #126
Вопрос : The neutral moleculas or negative ions bound to the central metal or ion in the coordination sphere called:
1) cation
2) ligands
3) complex ion
4) central atom
5) anion
Задание #127
Вопрос : …………….. is the most reactive metal.
1) Co 2) Mg 3) Pt 4) Ni 5) Fe
Задание #128
Вопрос : In which among the following are the noble metals soluble?
1) HCl
2) H2SO4
3) Aqua regia
4) NH4OH
5) Water gas
Задание #129
Вопрос : What happens when NH4OH is added to a solution of copper sulphate?
1) A deep blue solution of copper,s ammine complex is obtained
2) A deep blue solution is obtained
3) No change is observed
4) Blue precipitate of copper hydroxide is obtained
5) Black precipitate of copper oxide is obtained
Задание #130
Вопрос : Using IUPAC norms write the formula for the following: Hexaamminecobalt(III) sulphate
1) [Co(NH3)6 (SO4)3]
2) [Co(H2O)6]2 (SO4)3
3) [Co(NH3)6] SO4
4) [Co(NH3)6]2 (SO4)3
5) [Co(NH2)6]2 (SO4)3
Задание #131
Вопрос : Which of the following acids form three series of salts?
1) H3PO4
2) HPO3
3) H3PO2
4) H3BO3
5) H3PO2
Задание #132
Вопрос : Metal corrosion is ....
1) the formation of insoluble metal salt in the electrolyte solution
2) a conversion of chemical energy into electrical energy
3) a dissolution of metal in water
4) a dissolution of metal in acid
Дата добавления: 2018-09-20; просмотров: 261; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
