Table 2.7 Calculation for molecular mass of a gas mixture
| Gas Component | Percentage by Volume (Vi) | Component Molecular Mass (Mi) | MiVi/ 100 | Percentage by Mass |
| Methane | 83.2 | 16.04 | 13.35 | 67.6 |
| Ethane | 8.5 | 30.07 | 2.56 | 13.0 |
| Propane | 4.4 | 44.09 | 1.94 | 9.8 |
| Butane | 2.7 | 58.12 | 1.57 | 7.9 |
| Nitrogen | 1.2 | 28.02 | 0.34 | 1.7 |
| 100.00 | 19.76 | 100.00 | ||
| Mmix = 19.76 | ||||
|
Relative density of mixture = 19.76 / 29 = 0.681
| ||||
Vapour pressure of liquid mixtures
Dalton's Law of Partial Pressure states that when several gases occupy a common space, each behaves as though it occupies the space alone. The pressure which each gas exerts is called itspartial pressure and the total pressure exerted within the enclosing space is the sum of the partial pressures of the components.
Using Dalton's Law, it is possible to calculate the saturated vapour pressure of a mixture of liquids at a given temperature. The partial pressure exerted by the vapour of a liquid component, is equal to the product of the saturated vapour pressure of that component, if it existed alone at that temperature, multiplied by the mole fraction of the component in the liquid mixture. The total saturated vapour pressure of the mixture will be the sum of the partial pressures of each component.
Thus, Pmt = S (Pnt x Fn)
where Pmt is saturated vapour pressure of liquid mixture (m) at temperature (t)
Pnt is saturated vapour pressure of component (n) at temperature (t)
Fn is mole fraction of component (n) in liquid mixture. This is the mass of that
component divided by the mass of the whole mixture. For example, in
Table 2.7 the mole fraction of the gas mixture is given by:—
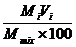
For example, for an LPG of the following composition at -40°C:—
| Component (n) | Mole Fraction in mixture (Fn) | SVP of component at-40°C (Pnt) (bar) | Partial Pressure of component at -40°C (Pnt x Fn) (bar) | Composition of vapour (Partial Pressure/SVP of mixture x 100) (% by volume) |
   Ethane
Propane
n-Butane
i-Butane Ethane
Propane
n-Butane
i-Butane
| 0.002 0.956 0.030 0.012 1.000 | 7.748 1.13 0.17 0.284 | 0.0155 1.0803 0.0051 0.0034 1.1043 | 1.4 97.8 0.5 0.3 100.0 |
| Saturated Vapour Pressure of mixture = 1.1043 bar.
| ||||
It is clear from the above example how the presence of a small amount of a very volatile component in the liquid mixture can add significantly to the vapour pressure. Because the components of the liquid mixture are in solution with each other, a low boiling component, such as the ethane in the above example, can remain in the liquid phase at temperatures well above the boiling point of the pure substance. However, the vapour phase will contain a higher proportion of such low boiling point material than does the liquid mixture.
2.18 BUBBLE POINTS AND DEW POINTS FOR MIXTURES
As outlined in 2.10 and illustrated in Figure 2.6, a pure liquid will commence to boil at a temperature depending upon the pressure above it. The liquid will continue to boil at

Figure 2.15 Equilibrium diagram for propane/butane mixtures
that temperature, provided the pressure is kept constant. On cooling superheated vapour to that same pressure, the vapour will become saturated at the same fixed temperature and will condense to liquid at that temperature. However, because of the differing volatilities of its components, a mixture of liquefied gases will behave differently. Thebubble point of a liquid mixture, at a given pressure, is defined as that temperature at which the liquid will begin to boil as the temperature rises. Thedew point of a vapour mixture, at a given pressure, is defined as the temperature at which the vapour begins to condense as the temperature decreases. For a liquid mixture in equilibrium with its vapour, the bubble point and the dew point are at different temperatures.
This behaviour can be represented on an equilibrium diagram. A typical example for propane/butane mixtures is shown in Figure 2.15. The diagram here gives vapour/ liquid equilibrium data for mixtures in terms of the mol percentage content in the liquid of the less-volatile component (butane). Equilibrium data must be related to a unique pressure and in this case the data is given for atmospheric pressure.
The two curves of Figure 2.15 show the bubble points and dew points of the mixture over a range from pure propane (zero percentage butane) to pure butane (100 per cent). It will be noted that at the two extremes, denoting either pure butane or pure propane, the bubble points and dew points become coincident. Interpreting the diagram, it can be seen that a liquid mixture of composition (A) will start to boil at its bubble point of -32.5°C but can only completely vaporise in equilibrium with its vapour provided the temperature rises to -10°C.
Similarly, a vapour mixture of composition (B) will start to condense at its dew point of -3°C but can only condense completely with a fall in temperature to -25°C.
A further use of such diagrams is the estimation of the differing proportions of the components in a liquid mixture and in its equilibrium vapour mixture. Taking again a liquid of composition (A), and assuming it is carried on a fully refrigerated ship at its initial bubble point of -32.5°C, at this temperature the vapour composition which will be in equilibrium with the liquid is given by (C).
2.19 RELIQUEFACTION AND ENTHALPY
2.19.1 Enthalpy
The enthalpy of a mass of a substance is a measure of its thermodynamic heat (or energy) content, whether the substance is liquid or vapour or a combination of the two. Within the Sl system it is measured in kiloJoules per kilogram. Enthalpy (H) is defined as:—
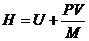
where H = enthalpy (kJ/kg)
U = internal energy (kJ/kg)
P = absolute pressure (kN/m2)
V = total volume of the system — liquid plus vapour (m3)
and M = mass in the system (kg)
[Note: Newtons = kg m/sec2; Joules = kg m2/sec2]
The total internal energy of a fluid is the thermodynamic energy attributable to its physical state. It includes sensible heat, latent heat, kinetic energy and potential energy. The PV term in the foregoing formula represents the energy available within a fluid due to pressure and volume.
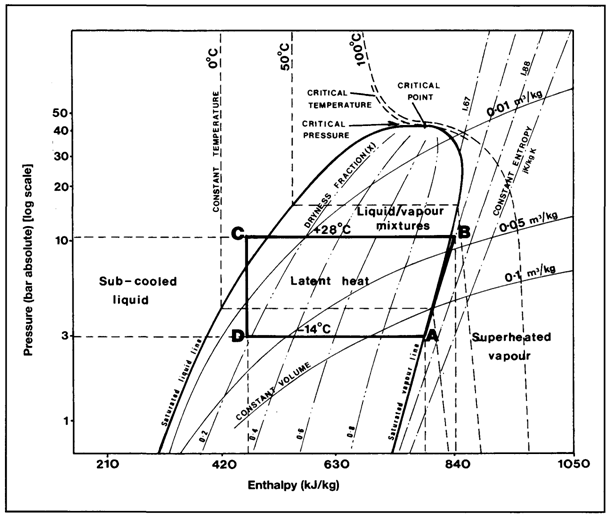
Absolute values of enthalpy are not normally of practical interest — it is the changes of enthalpy which are important in the thermodynamic analysis of a process. Accordingly, the enthalpy of a system is usually expressed from an arbitrarily chosen zero. Since a change in enthalpy expresses the total energy change in a fluid as it passes through any thermodynamic process, it is a useful unit for the analysis of energy changes. This is particularly so in cyclic processes involving compression, expansion, evaporation or condensation such as those encountered in the reliquefaction of boil-off vapours. In such processes, changes in kinetic energy and potential energy are negligible and thus enthalpy changes are calculable from well-established thermodynamic data. Tabular presentation of enthalpy changes for some liquefied gases are available but for many applications, the most widely used presentation is that found in Mollier diagrams. On one comprehensive chart, the Mollier diagram plots many different factors against absolute pressure (log scale) and enthalpy (linear scale). Mollier diagrams are available for a wide range of fluids, including all the liquefied gases, and should be available on board every LPG ship for the cargoes transported.
2.19.2 Refrigeration
Figure 2.16 depicts the principal features of the Mollier diagram for propane. In this diagram, the heat unit used is the kiloJoule. (The enthalpy scale is based upon the assumption of 419 kJ/kg at 0°C in the liquid phase.) The predominant feature of the diagram is the rounded conic shape of the liquid/vapour mixture area. This is enclosed
Дата добавления: 2018-02-28; просмотров: 2013; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
