Figure 2.10 Relationship between adiabatic and isothermal compression
Figure 2.10 is produced on similar axes as Figure 2.9(a). However, Figure 2.10 includes two isothermal lines — one for a low temperature (T1) and one for a higher temperature (T2). For a compressor, as the changes lie closer to the adiabatic line than the isothermal line, it is usual to assume an adiabatic change in such cases.
As covered at the beginning of this section under the discussion on Boyle's Law, the equation for an isothermal compression is:—
PV = constant It may be of interest to note that the equation for the adiabatic compression is:
PVk = constant
where 'k' is the ratio of principal specific heats for the substance. This is the ratio of specific heat of the liquid divided by the specific heat of the vapour.
2.15 SATURATED VAPOUR PRESSURE
In 2.14, discussion centred on pure gases isolated from their liquids. In this section, attention is given to gases in contact with their own liquids. It is in this respect that the concept of saturated vapour pressure (SVP) becomes important.
Vapour in the space above a liquid is in constant motion. Molecules near the liquid surface are constantly leaving to enter the vapour-phase and molecules in the vapour are returning to the liquid-phase. The vapour space is said to be unsaturated if it can accept more vapour from the liquid at its current temperature. A saturated vapour is a vapour in equilibrium with its liquid at that temperature. In that condition, the vapour space cannot accept any further ingress from the liquid without a continuous exchange of molecules taking place between vapour and liquid.
The pressure exerted by a saturated vapour at a particular temperature is called the saturated vapour pressure of that substance at that temperature. Various methods exist for the measurement of saturated vapour pressures and one is illustrated in Figure 2.11. This apparatus consists of a barometric tube (C) which is filled with mercury, inverted and immersed in a mercury reservoir (A). The space above the mercury is a virtual vacuum (B). The height of mercury (X) is a measure of atmospheric pressure. A small amount of the liquid under test is introduced into the mercury barometer and this rises to the vacuum space. Here it partially vaporises and exerts its saturated vapour pressure. This vapour pressure pushes the mercury down the barometer tube to a new level (Y). The saturated vapour pressure exerted by the test liquid is shown by the difference between the heights of the mercury column X and Y and, in this case, is usually expressed in millimetres of mercury.
If the mercury column containing the liquid under test is heated, then the mercury level will fall further, indicating that the saturated vapour pressure has increased with increasing temperature. It is possible by this means to determine the saturated vapour pressure for the liquid under test at various temperatures.
Evaporation is a phenomenon where the faster-moving molecules escape from the surface of a liquid. However, when boiling occurs, it takes place in the body of the liquid. This happens when the external vapour pressure is equal to the pressure of the liquid. By varying the pressure above the liquid the liquid boils at different
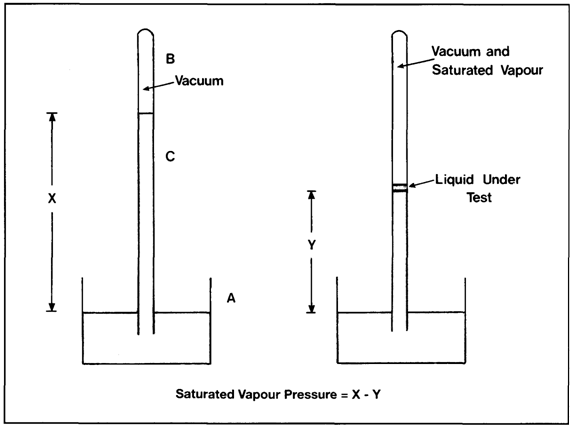
Figure 2.11 Barometric method for measuring saturated vapour pressure

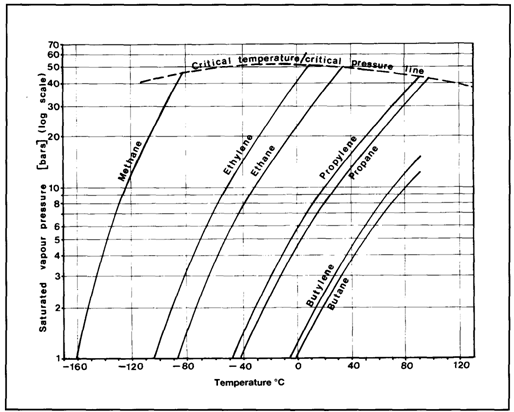
Figure 2.12 Characteristics of propane
Дата добавления: 2018-02-28; просмотров: 689; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
