Figure 2.6 Temperature/heat diagram for varying states of matter
Table 2.5 Physical properties of gases
 Gas Gas
| Atmospheric boiling point (°C) | Critical temperature (°C) | Critical pressure (bars, absolute) | Condensing ratio dm3 liquid 1m3 gas | Liquid relative density at Atm. Boiling Pt. (Water = 1) | Vapour relative density (Air = 1) |
| Methane | -161.5 | -82.5 | 44.7 | 0.804 | 0.427 | 0.554 |
| Ethane | - 88.6 | 32.1 | 48.9 | 2.453 | 0.540 | 1.048 |
| Propane | - 42.3 | 96.8 | 42.6 | 3.380 | 0.583 | 1.55 |
| n-Butane | - 0.5 | 153 | 38.1 | 4.32 | 0.600 | 2.09 |
| i-Butane | - 11.7 | 133.7 | 38.2 | 4.36 | 0.596 | 2.07 |
| Ethylene | -103.9 | 9.9 | 50.5 | 2.20 | 0.570 | 0.975 |
| Propylene | - 47.7 | 92.1 | 45.6 | 3.08 | 0.613 | 1.48 |
| a-Butylene | - 6.1 | 146.4 | 38.9 | 4.01 | 0.624 | 1.94 |
| g-Butylene | - 6.9 | 144.7 | 38.7 | 4.00 | 0.627 | 1.94 |
| Butadiene | - 5.0 | 161.8 | 43.2 | 3.81 | 0.653 | 1.88 |
| Isoprene | 34 | 211.0 | 38.5 | 0.67 | 2.3 | |
| Vinyl chloride | - 13.8 | 158.4 | 52.9 | 2.87 | 0.965 | 2.15 |
| Ethylene oxide | 10.7 | 195.7 | 74.4 | 2.13 | 0.896 | 1.52 |
| Propylene oxide | 34.2 | 209.1 | 47.7 | 0.830 | 2.00 | |
| Ammonia | -33.4 | 132.4 | 113.0 | 1.12 | 0.683 | 0.597 |
| Chlorine | - 34 | 144 | 77.1 | 2.03 | 1.56 | 2.49 |
Fusion or solidification occurs at a specific temperature for each substance and this temperature is virtually independent of the pressure. However, vaporisation or condensation of a pure substance occurs at a temperature which varies widely depending upon the pressure exerted. It should also be noted that the latent heat of vaporisation varies with pressure. Figure 2.6 illustrates these temperature/heat relationships as a substance is heated or cooled through its three states: here the temperatures of fusion or solidification (A) and of vaporisation or condensation (B) are shown.
For liquefied gases, the solid state is not of concern since this can only occur at temperatures well below those at which such gases are carried. However, temperatures, pressures and latent heats of vaporisation are of fundamental importance. This data may be presented in graphical form such as Figure 2.7 which gives data for methane covering absolute vapour pressure (P), liquid density (y'), saturated vapour
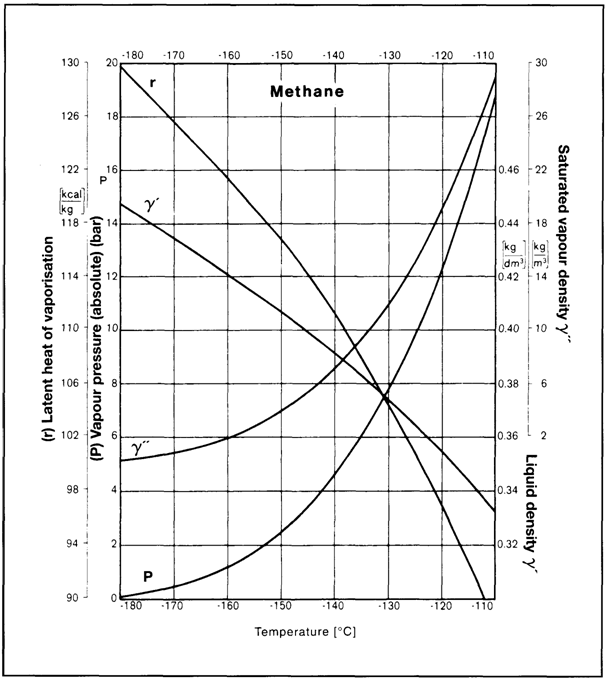
Figure 2.7 Characteristics of methane
density (y") and latent heat of vaporisation (r) against temperature. Similar graphical presentations of these properties are available elsewhere for all liquefied gases carried by sea.
2.10.2 Spillage of liquefied gas
It is convenient here, against the background of the preceding paragraphs, to consider what happens when a liquefied gas is spilled. Firstly, consider the escape from its containment of a fully refrigerated liquid. Here the liquid is already at or near atmospheric pressure but, on escape, it is brought immediately into contact with the ground or sea at ambient temperature. The temperature difference between the cold liquid and the material it contacts provides an immediate heat transfer into the liquid, resulting in the rapid evolution of vapour. If the spill is lying in a pool on the ground, the removal of heat from the ground beneath narrows the temperature difference. Eventually, temperature differences stabilise and the rate of evaporation continues at a lower level. Under these conditions, the liquid will continue to boil until completely evaporated. For spills on the sea, the strong convection currents in the water may maintain the initial temperature difference and evaporation will probably continue at the higher initial rate. In this case, the large quantities of cold vapour produced from the liquid will diffuse into the atmosphere and cause condensation of the water vapour in the air. By this process, a visible vapour cloud is formed which is white in colour.
Initial spillage of a liquefied gas from a pressure vessel behaves differently to that described above. In this case the liquid, on escape, is at a temperature close to ambient. However, the high pressure at release, quickly falls to ambient and this results in extremely rapid vaporisation, the necessary heat being taken primarily from the liquid itself. This is calledflash evaporation and, depending upon the change in pressure, much of the liquid may flash-off in this way. By this means any remaining liquid is cooled rapidly to its refrigerated temperature (and even lower) at atmospheric pressure. High-pressure liquids escaping in this way cause much of it to spray into the atmosphere as small droplets. These droplets take heat from the atmosphere and condense the water vapour in the air to form a white visible cloud. The liquid droplets soon vaporise to gas and in the process causes further cooling, so maintaining the white cloud formation for longer. Thereafter, any remaining liquid pools attain an equilibrium temperature and evaporate, as described in the preceding paragraph, until wholly vaporised.
The hazard introduced by the escape of vapour into the atmosphere is that, on mixing with the air, it becomes flammable. The white vapour cloud so formed can give warning of the presence of a hazardous condition but it should be noted that the flammable extent of the gas cloud will not necessarily coincide with the visible cloud.
Apart from the hazards introduced by vapour-in-air mixtures, the cold liquid can cause frostbite on human tissue and may convert metals to a brittle state. Furthermore, on exposure to air it is likely that a liquefied gas will become sub-cooled to a temperature below its atmospheric boiling point (see also Chapter Ten).
2.11 PRINCIPLES OF REFRIGERATION
The principles of heat transfer, evaporation and condensation are applied in refrigeration. Figure 2.8 illustrates the basic components and operating cycle of a simple refrigerator. Cold liquid refrigerant is vaporised in an evaporator which, being colder than its surroundings, draws in heat to provide the latent heat of vaporisation. The cool
vapour is drawn off by a compressor which raises both the pressure and the temperature of the vapour and passes it to the condenser. Here, the vapour is condensed to a high-pressure liquid and the sensible heat from desuperheating, together with latent heat of condensation, is removed by means of the condenser coolant, which is warmed in the process. The high-pressure liquid then passes through an expansion valve to the low-pressure side of the refrigerator and, in doing so, flash evaporates to a two-phase mixture of cold liquid and vapour. This mixture then passes to the evaporator (cargo tank) to complete the cycle.
In considering Figure 2.8, if:
Q1 is the heat flow rate from the surroundings into the evaporator
Q2 is the heat-rate equivalent of work done on the vapour by the compressor, and
Q3 is the heat-rate rejected by the condenser
then, if the system were 100 per cent efficient:—
Q1 + Q2= Q3

Дата добавления: 2018-02-28; просмотров: 775; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
