Hormones of the anterior pituitary. The chemical nature and the effect on the metabolism.
Enzymes isomerases
In biochemistry, an isomerase is an enzyme that catalyses the structural rearrangement of isomers. Isomerases thus catalyze reactions of the form
Nomenclature
The names of isomerases are formed as "substrate" isomerase" (for example, enoyl CoA isomerase), or as "substrate" "type of isomerase" (for example, phosphoglucomutase).
Classification
Isomerases have their own EC classification of enzymes: EC 5. Isomerases can be further classified into six subclasses:
* includes enzymes that catalyze racemization (racemases) and epimerization (epimerases)
* includes enzymes that catalyze the isomerization of geometric isomers (cis-trans isomerases)
* includes intramolecular oxidoreductases
* includes intramolecular transferases (mutases)
* includes intramolecular lyases
* includes other isomerases (including topoisomerases)
Lyase enzymes
In biochemistry, a lyase is an enzyme that catalyzes the breaking (an "elimination" reaction) of various chemical bonds by means other than hydrolysis (a "substitution" reaction) and oxidation, often forming a new double bond or a new ring structure. The reverse reaction is also possible (called a "Michael addition”). For example, an enzyme that catalyzed this reaction would be a lyase:
ATP → cAMP + PPi
Lyases differ from other enzymes in that they require only one substrate for the reaction in one direction, but two substrates for the reverse reaction.
Lygase enzymes
In biochemistry, ligase (from the Latin verb ligāre — "to bind" or "to glue together") is an enzyme that can catalyze the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group dependent to one of the larger molecules or the enzyme catalyzing the linking together of two compounds, e.g., enzymes that catalyze joining of C-O, C-S, C-N, etc. In general, a ligase catalyzes the following reaction:
Ab + C → A–C + b
or sometimes
Ab + cD → A–D + b + c
where the lowercase letters signify the small, dependent groups.
15 Vitamins. History of discabery. Vitamin deficiency – hypovitaminosis and hypervitaminosis.
A vitamin (US /ˈvaɪtəmɪn/ or UK /ˈvɪtəmɪn/) is an organic compound required by an organism as a vital nutrient in limited amounts.[1] An organic chemical compound (or related set of compounds) is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on the circumstances and on the particular organism. For example, ascorbic acid (vitamin C) is a vitamin for humans, but not for most other animals. Supplementation is important for the treatment of certain health problems but there is little evidence of benefit when used by those who are otherwise healthy
|
|
|
In 1747, the Scottish surgeon James Lind discovered that citrus foods helped prevent scurvy, a particularly deadly disease in which collagen is not properly formed, causing poor wound healing, bleeding of the gums, severe pain, and death.[39] In 1753, Lind published his Treatise on the Scurvy, which recommended usinglemons and limes to avoid scurvy, which was adopted by the British Royal Navy. This led to the nicknameLimey for sailors of that organization. Lind's discovery, however, was not widely accepted by individuals in the Royal Navy's Arctic expeditions in the 19th century, where it was widely believed that scurvy could be prevented by practicing good hygiene, regular exercise, and maintaining the morale of the crew while on board, rather than by a diet of fresh food.[39] As a result, Arctic expeditions continued to be plagued by scurvy and other deficiency diseases. In the early 20th century, when Robert Falcon Scott made his two expeditions to the Antarctic, the prevailing medical theory at the time was that scurvy was caused by "tainted" canned food.[39]
During the late 18th and early 19th centuries, the use of deprivation studies allowed scientists to isolate and identify a number of vitamins. Lipid from fish oil was used to cure rickets in rats, and the fat-soluble nutrient was called "antirachitic A". Thus, the first "vitamin" bioactivity ever isolated, which cured rickets, was initially called "vitamin A"; however, the bioactivity of this compound is now called vitamin D.[41] In 1881, Russian surgeon Nikolai Lunin studied the effects of scurvy while at the University of Tartu in present-day Estonia.[42] He fed mice an artificial mixture of all the separate constituents of milk known at that time, namely the proteins,fats,carbohydrates, and salts. The mice that received only the individual constituents died, while the mice fed by milk itself developed normally. He made a conclusion that "a natural food such as milk must therefore contain, besides these known principal ingredients, small quantities of unknown substances essential to life."[42] However, his conclusions were rejected by other researchers when they were unable to reproduce his results. One difference was that he had used table sugar (sucrose), while other researchers had used milk sugar (lactose) that still contained small amounts of vitamin B.
|
|
|
In east Asia, where polished white rice was the common staple food of the middle class,beriberi resulting from lack of vitamin B1 was endemic. In 1884, Takaki Kanehiro, a British trained medical doctor of the Imperial Japanese Navy, observed that beriberi was endemicamong low-ranking crew who often ate nothing but rice, but not among officers who consumed a Western-style diet. With the support of the Japanese navy, he experimented using crews of two battleships; one crew was fed only white rice, while the other was fed a diet of meat, fish, barley, rice, and beans. The group that ate only white rice documented 161 crew members with beriberi and 25 deaths, while the latter group had only 14 cases of beriberi and no deaths. This convinced Takaki and the Japanese Navy that diet was the cause of beriberi, but mistakenly believed that sufficient amounts of protein prevented it.[43]That diseases could result from some dietary deficiencies was further investigated byChristiaan Eijkman, who in 1897 discovered that feeding unpolished rice instead of the polished variety to chickens helped to prevent beriberi in the chickens. The following year,Frederick Hopkins postulated that some foods contained "accessory factors" — in addition to proteins, carbohydrates, fats etc. — that are necessary for the functions of the human body.[39] Hopkins and Eijkman were awarded the Nobel Prize for Physiology or Medicine in 1929 for their discovery of several vitamins.[44]
|
|
|
In 1910, the first vitamin complex was isolated by Japanese scientist Umetaro Suzuki, who succeeded in extracting a water-soluble complex of micronutrients from rice bran and named it aberic acid (later Orizanin). He published this discovery in a Japanese scientific journal.[45]When the article was translated into German, the translation failed to state that it was a newly discovered nutrient, a claim made in the original Japanese article, and hence his discovery failed to gain publicity. In 1912 Polish biochemist Casimir Funk isolated the same complex of micronutrients and proposed the complex be named "vitamine" (from "vital amine", reportedly suggested by Max Nierensteina friend and reader of Biochemistry at Bristol University[46]).[47] The name soon became synonymous with Hopkins' "accessory factors", and, by the time it was shown that not all vitamins are amines, the word was already ubiquitous. In 1920, Jack Cecil Drummond proposed that the final "e" be dropped to deemphasize the "amine" reference, after researchers began to suspect that not all "vitamines" (in particular, vitamin A) have an amine component.[43]
In 1930, Paul Karrer elucidated the correct structure for beta-carotene, the main precursor of vitamin A, and identified other carotenoids. Karrer and Norman Haworth confirmed Albert Szent-Györgyi's discovery of ascorbic acid and made significant contributions to the chemistry of flavins, which led to the identification of lactoflavin. For their investigations on carotenoids, flavins and vitamins A and B2, they both received the Nobel Prize in Chemistry in 1937.[48]
In 1931, Albert Szent-Györgyi and a fellow researcher Joseph Svirbely suspected that "hexuronic acid" was actually vitamin C, and gave a sample to Charles Glen King, who proved its anti-scorbutic activity in his long-established guinea pig scorbutic assay. In 1937, Szent-Györgyi was awarded the Nobel Prize in Physiology or Medicine for his discovery. In 1943, Edward Adelbert Doisy and Henrik Damwere awarded the Nobel Prize in Physiology or Medicine for their discovery of vitamin K and its chemical structure. In 1967, George Wald was awarded the Nobel Prize (along with Ragnar Granit and Haldan Keffer Hartline) for his discovery that vitamin A could participate directly in a physiological process.[44]
|
|
|
Hypervitaminosis E is a state of vitamin E toxicity. Because vitamin E can act as an anticoagulant and may increase the risk of bleeding problems, many agencies have set a tolerable upper intake levels (UL) for vitamin E at 1,000 mg (1,500 IU) per day.[1] This UL was established due to an increased incidence of hemorrhaging with higher doses of supplemental vitamin E. Doses of vitamin E above the UL can also potentiate the antiplatelet effects of certain drugs such as anti-coagulant medications and aspirin, which can cause life-threatening symptoms in ill patients. Hypervitaminosis E may also counteract vitamin K, leading to a vitamin K deficiency.
Hypovitaminosis A is a common problem in pet turtles caused by inadequate Vitamin A intake in the diet. The body requires Vitamin A to form healthy skin, mucous membranes and ducts (small tubes that conduct fluids such as urine, saliva, or bile) within organs such as the kidneys and salivary glands. When Vitamin A is insufficient in the body, squamous metaplasia (an abnormal growth and thickening of cells) occurs, which disrupts the normal function of the skin or organs, most frequently by blocking fluid flow through ducts.
Hypovitaminosis A is most commonly seen in juvenile semi-aquatic turtles, like painted turtles or red eared sliders, or box turtles greater than six months of age. It is seen very rarely in tortoises because they are normally fed a diet that is rich in dark green and yellow vegetables.
16 A vitamin
Vitamin A is the collective name for a group of fat-soluble vitamins. The most useable form of the vitamin is retinol, often called preformed vitamin A as it is the active form in the body. Retinol is chemically a pale yellow crystalline solid. Vitamin A palmitate
(retinyl palmitate) and vitamin A acetate (retinyl acetate) are the principal forms used as nutritional supplements. Retinyl palmitate is a more stable version of retinol, however, because the skin has to further break downretinyl palmitate, much higher concentrations are required to provide the similar benefits. When choosing between the two, it is better to go with the formula containing retinol rather than retinyl palmitate. The precursors of vitamin A (retinol) are the carotenoids (most commonlybeta-carotene). Retinol, retinal, retinoic acid, and related compounds are known as retinoids. Retinal can be converted by the body to retinoic acid, the form of vitamin A known to affect gene transcription. Beta-carotene and other carotenoids that can be converted by the body into retinol are referred to as provitamin A carotenoids.
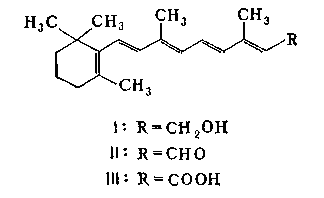
17 D vitamin
Vitamin D is a group of fat-soluble secosteroids responsible for enhancing intestinal absorption of calcium and phosphate. In humans, the most important compounds in this group are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol).[1]Cholecalciferol and ergocalciferol can be ingested from the diet and from supplements.[1][2][3] The body can also synthesize vitamin D (specifically cholecalciferol) in the skin, from cholesterol, when sun exposure is adequate (hence its nickname, the "sunshine vitamin").
Although vitamin D is commonly called a vitamin, it is not actually an essential dietary vitamin in the strict sense, as it can be synthesized in adequate amounts by most mammals exposed to sunlight. A substance is only classified as an essential vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from its diet. In common with other compounds commonly called vitamins, vitamin D was nevertheless discovered in an effort to find the dietary substance lacking in a disease, namely rickets, the childhood form of osteomalacia.[4] Additionally, like other compounds called vitamins, in the developed world, vitamin D is added to staple foods, such as milk, to avoid disease due to deficiency.

18 E vitamin
Vitamin E refers to a group of eight fat-soluble compounds that include both tocopherolsand tocotrienols.[1] Of the many different forms of vitamin E, γ-tocopherol is the most common in the North American diet.[2] γ-Tocopherol can be found in corn oil, soybean oil, margarine, and dressings.[3][4] α-tocopherol, the most biologically active form of vitamin E, is the second-most common form of vitamin E in the diet. This variant can be found most abundantly in wheat germ oil, sunflower, and safflower oils.[4][5] As a fat-solubleantioxidant, it stops the production of reactive oxygen species formed when fat undergoes oxidation.[6][7][8] Amounts over 1,000 mg (1,500 IU) per day[9] are called Hypervitaminosis E, as they may increase the risk of bleeding problems and vitamin K deficiency.

19 K vitamin. Vicasol. F vitamin
Vitamin K is a group of structurally similar, fat-soluble vitamins that the human body needs for post-translational modification of certain proteins required for blood coagulation, and in metabolic pathways in bone and other tissue. They are 2-methyl-1,4-naphthoquinone (3-) derivatives. This group of vitamins includes two natural vitamers:vitamin K1 and vitamin K2.[1]
Vitamin K1, also known as phylloquinone, phytomenadione, or phytonadione, is synthesized by plants, and is found in highest amounts in green leafy vegetables because it is directly involved in photosynthesis. It may be thought of as the "plant form" of vitamin K. It is active in animals and may perform the classic functions of vitamin K in animals, including its activity in the production of blood-clotting proteins. Animals may also convert it to vitamin K2. The function of vitamin K1 in the cell is to add a carboxylic acid functional group to a glutamate amino acid residue in a protein, to form a gamma-carboxyglutamate (Gla) residue. This is a somewhat uncommon posttranslational modification of the protein, which is then known as a "Gla protein." The presense of two -COOH (carboxylate) groups on the same carbon in the gamma-carboxyglutamate residue allows it to chelate calcium ion. The binding of calcium ion in this way very often triggers the function or binding of Gla-protein enzymes, such as the so-called vitamin K dependent clotting factors discussed below.

ВитаминФ Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health but cannot synthesize them.[1] The term "essential fatty acid" refers to fatty acids required for biological processes but does not include the fats that only act as fuel.
Only two fatty acids are known to be essential for humans: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).[2][3] Some other fatty acids are sometimes classified as "conditionally essential," meaning that they can become essential under some developmental or disease conditions; examples include docosahexaenoic acid (an omega-3 fatty acid) and gamma-linolenic acid (an omega-6 fatty acid).
When the two EFAs were discovered in 1923, they were designated "vitamin F", but in 1929, research on rats showed that the two EFAs are better classified as fats rather than vitamins.[3]

20 B1 vitamin
Thiamine or thiamin or vitamin B1 (/ˈθaɪ.əmɨn/ thy -ə-min), named as the "thio-vitamine" ("sulfur-containing vitamin") is a water-soluble vitamin of the B complex. First named aneurin for the detrimental neurological effects if not present in the diet, it was eventually assigned the generic descriptor name vitamin B1. Its phosphate derivatives are involved in many cellular processes. The best-characterized form is thiamine pyrophosphate (TPP), acoenzyme in the catabolism of sugars and amino acids. Thiamine is used in the biosynthesis of the neurotransmitter acetylcholine and gamma-aminobutyric acid (GABA). In yeast, TPP is also required in the first step of alcoholic fermentation.
All living organisms use thiamine, but it is synthesized only in bacteria, fungi, and plants.Animals must obtain it from their diet, and thus, for them, it is an essential nutrient. Insufficient intake in birds produces a characteristic polyneuritis. In mammals, deficiency results in Korsakoff's syndrome, optic neuropathy, and a disease called beriberi that affects the peripheral nervous system (polyneuritis) and/or the cardiovascular system. Thiamine deficiency has a potentially fatal outcome if it remains untreated.[1] In less severe cases, nonspecific signs include malaise, weight loss, irritability and confusion

21 B2 vitamin
Riboflavin, also known as vitamin B2 is an easily absorbed colored micronutrient with a key role in maintaining health in humans and other animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a wide variety of cellular processes. It plays a key role in energy metabolism, and for the metabolism of fats, ketone bodies, carbohydrates, and proteins.
Milk, cheese, leaf vegetables, liver, kidneys, legumes, yeast, mushrooms, and almonds[2]are good sources of vitamin B2.
The name "riboflavin" comes from "ribose" (the sugar whose reduced form, ribitol, forms part of its structure) and "flavin", the ring-moiety which imparts the yellow color to the oxidized molecule (from Latin flavus, "yellow"). The reduced form, which occurs in metabolism along with the oxidized form, is colorless.
Riboflavin is best known visually as the vitamin which imparts the orange color to solid B-vitamin preparations, the yellow color to vitamin supplement solutions, and the unusual fluorescent-yellow color to the urine of persons who supplement with high-dose B-complex preparations.

22 PP vitamin
Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide ofnicotinic acid (vitamin B3 / niacin). Nicotinamide is a water-soluble vitamin and is part of the vitamin B group. Nicotinic acid, also known as niacin, is converted to nicotinamide in vivo, and, though the two are identical in their vitamin functions, nicotinamide does not have the same pharmacological and toxic effects of niacin, which occur incidental to niacin's conversion. Thus nicotinamide does not reduce cholesterol or cause flushing,[1]although nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[2] In cells, niacin is incorporated into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), although the pathways for nicotinamide and nicotinic acid are very similar. NAD+ and NADP+ are coenzymes in a wide variety of enzymatic oxidation-reduction reactions.[3] It's produced by the aqueous aminolysis of 3-cyanopyridine (nicotinonitrile) and subsequent crystallization.

Вопрос
Vitamin B6 is a water-soluble vitamin and is part of the vitamin B complex group. Several forms of the vitamin are known, but pyridoxal phosphate (PLP) is the active form and is a cofactor in many reactions of amino acid metabolism, including transamination, deamination, and decarboxylation. PLP also is necessary for the enzymatic reaction governing the release of glucose from glycogen.
In 1934 a Hungarian physician Paul Gyorgy discovered a substance that was able to cure a skin disease in rats (dermititis acrodynia). He named this substance vitamin B6.[1][2] In 1938, Samuel Lepkovsky isolated vitamin B6 from rice bran. Harris and Folkers in 1939 determined the structure of pyridoxine, and, in 1945, Snell was able to show the two forms of vitamin B6, pyridoxal and pyridoxamine. Vitamin B6 was named pyridoxine to indicate its structural homology to pyridine. All three forms of vitamin B6 are precursors of an activated compound known as pyridoxal 5'-phosphate (PLP), which plays a vital role as the cofactor of a large number of essential enzymes in the human body.
Pyridoxal phosphate, the metabolically active form of vitamin B6, is involved in many aspects of macronutrient metabolism, neurotransmitter synthesis, histamine synthesis, hemoglobin synthesis and function and gene expression. Pyridoxal phosphate generally serves as a coenzyme for many reactions and can help facilitate decarboxylation, transamination, racemization, elimination, replacement and beta-group interconversion reactions.[4] The liver is the site for vitamin B6 metabolism.
Vitamin B6 is involved in the following metabolic processes:
· amino acid, glucose and lipid metabolism
· neurotransmitter synthesis
· histamine synthesis
· hemoglobin synthesis and function
· gene expression
24 вопрос
Vitamin C or L-ascorbic acid, or simply ascorbate (the anion of ascorbic acid), is an essential nutrient for humans and certain other animal species. Vitamin C refers to a number of vitamers that have vitamin C activity in animals, including ascorbic acid and its salts, and some oxidized forms of the molecule like dehydroascorbic acid. Ascorbate and ascorbic acid are both naturally present in the body when either of these is introduced into cells, since the forms interconvert according to pH.
The name vitamin C always refers to the L -enantiomer of ascorbic acid and its oxidized forms
Vitamin C acts as an electron donor for eight different enzymes:[49]
· Three enzymes (prolyl-3-hydroxylase, prolyl-4-hydroxylase, and lysyl hydroxylase) that are required for the hydroxylation proline and lysine in the synthesis of collagen hydroxylation.[50][51][52]These reactions add hydroxyl groups to the amino acids proline or lysine in the collagen molecule via prolyl hydroxylase and lysyl hydroxylase, both requiring vitamin C as a cofactor. Hydroxylation allows the collagen molecule to assume its triple helix structure, and thus vitamin C is essential to the development and maintenance of scar tissue, blood vessels, andcartilage.[40]
· Two enzymes (ε-N-trimethyl-L-lysine hydroxylase and γ-butyrobetaine hydroxylase) that are necessary for synthesis of carnitine.[53][54] Carnitine is essential for the transport of fatty acids intomitochondria for ATP generation.
· The remaining three enzymes have the following functions in common, but have other functions as well:
· dopamine beta hydroxylase participates in the biosynthesis of norepinephrine from dopamine.[55][56]
· another enzyme (peptidylglycine alpha-amidating monooxygenase) adds amide groups to peptide hormones, greatly increasing their stability.[57][58]
· 4-hydroxyphenylpyruvate dioxygenase modulates tyrosine metabolism.[59][60
25 вопрос
Folic acid (also known as folate, vitamin M, vitamin B9,[3] vitamin Bc [4] (or folacin), pteroyl-L-glutamic acid, and pteroyl-L-glutamate [5]) are forms of the water-soluble vitamin B9. Folate is composed of the aromatic pteridine ring linked to para-aminobenzoic acid and one or more glutamate residues. Folic acid is itself not biologically active, but its biological importance is due to tetrahydrofolate and other derivatives after its conversion to dihydrofolic acid in the liver.[6]
· Molecular Formula: C19H19N7O6
· Heart disease [edit]
· Taking folic acid does not reduce cardiovascular disease even though it reduces homocysteine levels.[34]
· Folic acid supplements consumed before and during pregnancy may reduce the risk of heart defects in infants,[35] and may reduce the risk for children to develop metabolic syndrome.[36]
· In one small study of folic acid as secondary prevention of cardiac events following unstable angina or non-ST-elevation myocardial infarction involving 240 patients, outcomes were no better, and may actually have been worse, in the group receiving folate, vitamin B-12 and vitamin B-6 than in the placebo group.[37]
· A 2005 study found that 5 mg of folate daily over a three-week period reduced elevated pulse pressure, which is an independent risk factor for atherosclerosis and other undesirable cardiac and whole body effects. The reduction was by 4.7 mm of Hg compared with a placebo. The study concluded that folic acid is an effective supplement that targets large artery stiffness and may reduce risks of the development of isolated systolic hypertension.[38]
· Pantothenic acid, also called pantothenate or vitamin B5 (a B vitamin), is a water-soluble vitamin discovered by Roger J. Williams in 1933.[2] For many animals, pantothenic acid is an essential nutrient. Animals require pantothenic acid to synthesize coenzyme-A (CoA), as well as to synthesize and metabolize proteins, carbohydrates, and fats.
· Pantothenic acid is the amide between pantoic acid and β-alanine. Its name derives from the Greek pantothen (πάντοθεν) meaning "from everywhere" and small quantities of pantothenic acid are found in nearly every food, with high amounts in whole-grain cereals, legumes, eggs, meat, royal jelly,avocado, and yogurt.[3] It is commonly found as its alcohol analog, the provitamin panthenol, and as calcium pantothenate. Pantothenic acid is an ingredient in some hair and skin care products.[4]
Testicular torsion[edit]
Testicular torsion can severely affect fertility if it occurs.[20] One study on a rat model indicated a treatment of 500 mg of dexpanthenol/kg body weight 30 minutes prior to detorsion can greatly decrease the risk of infertility after torsion.[20] Pantothenic acid has the ability to spare reduced glutathione levels.[21] Reactive oxygen species play a role in testicular atrophy, which glutathione counteracts.[20]
Diabetic ulceration[edit]
Foot ulceration is a problem commonly associated with diabetes, which often leads to amputation.[22] A preliminary study completed by Abdelatif, Yakoot and Etmaan indicated that perhaps a royal jelly and panthenol ointment can help cure the ulceration.[22] People with foot ulceration or deep tissue infection in the study had a 96% and 92% success rate of recovery.[22] While these results appear promising, they need to be validated, as this was a pilot study; it was not a randomized, placebo-controlled, double-blind study.[22]
Hypolipidemic effects[edit]
Pantothenic acid derivatives, panthenol, phosphopantethine and pantethine, have also been seen to improve the lipid profile in the blood and liver.[23] In this mouse model, they injected 150 mg of the derivative/kg body weight.[23] All three derivatives were able to effectively lower low-density lipoprotein (LDL), as well as triglyceride (TG) levels; panthenol was able to lower total cholesterol, and pantethine was able to lower LDL-cholesterol in the serum.[23] The decrease in LDL is significant, as it is related to a decrease the risk of myocardial infarction and stroke.[8] In the liver, panthenol was the most effective, as it lowered TG, total cholesterol, free cholesterol and cholesterol-ester levels.
Вопрос
Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brainand nervous system, and for the formation of blood. It is one of the eight B vitamins. It is normally involved in the metabolism of every cell of the human body, especially affecting DNA synthesis and regulation, but also fatty acid synthesis and energy production. Neither fungi, plants, nor animals are capable of producing vitamin B12. Only bacteria and archaea have the enzymes required for its synthesis, although many foods are a natural source of B12 because of bacterial symbiosis. The vitamin is the largest and most structurally complicated vitamin and can be produced industrially only through bacterial fermentation-synthesis.
Vitamin B12 consists of a class of chemically related compounds (vitamers), all of which have vitamin activity. It contains the biochemically rare element cobalt. Biosynthesis of the basic structure of the vitamin is accomplished only by bacteria (which usually produce hydroxocobalamin), but conversion between different forms of the vitamin can be accomplished in the human body. A common semi-synthetic form of the vitamin,cyanocobalamin, does not occur in nature, but is produced from bacterial hydroxocobalamin and then used in many pharmaceuticals and supplements, and as a food additive, because of its stability and lower production cost. In the body it is converted to the human physiological formsmethylcobalamin and adenosylcobalamin, leaving behind the cyanide, albeit in minimal concentration. More recently, hydroxocobalamin, methylcobalamin, and adenosylcobalamin can be found in more expensive pharmacological products and food supplements. The extra utility of these is currently debated.
Vitamin B12 was discovered from its relationship to disease pernicious anemia, which is an autoimmune disease in which parietal cells of the stomach responsible for secreting intrinsic factor are destroyed. Intrinsic factor is crucial for the normal absorption of B12, so a lack of intrinsic factor, as seen in pernicious anemia, causes a vitamin B12 deficiency. Many other subtler kinds of vitamin B12 deficiency and their biochemical effects have since been elucidated.[1]Vitamin B12 is used to treat vitamin B12 deficiency, cyanide poisoning, and hereditary deficiency of transcobalamin II.[9] It is given as part of the Schilling test for detecting pernicious anemia.[9]For cyanide poisoning, a large amount may be given intravenously and sometimes in combination with sodium thiosulfate.[10] The mechanism of action is straightforward: the hydroxycobalamin hydroxide ligand is displaced by the toxic cyanide ion, and the resulting harmless B12 complex is excreted in urine. In the United States, the Food and Drug Administration approved (in 2006) the use of hydroxocobalamin for acute treatment of cyanide poisoning.[11]High vitamin B12 level in elderly individuals may protect against brain atrophy or shrinkage associated with Alzheimer's disease and impaired cognitive function.[12]High-dose administration of Vitamin B12 has been additionally validated to stimulate the activity of the body's TH1 suppressor T-Cells, which then down-regulates the over-production of the allergen antibody IgE in allergic individuals
Вопрос
Thyroxine-binding globulin (TBG) binds thyroid hormone in circulation. It is one of three proteins (along with transthyretin and albumin) responsible for carrying the thyroid hormones thyroxine(T4) and 3,5,3’-triiodothyronine (T3) in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3, but is present in the lowest concentration. Despite its low concentration, TBG carries the majority of T4 in the blood. Due to the very low concentration of T4 & T3 in the blood, TBG is rarely more than 25% saturated with its ligand. Unlike transthyretin and albumin, TBG has a single binding site for T4/T3. TBG is synthesized primarily in the liver as a 54 kDa protein. In terms of genomics, TBG is a serpin; however, it has no inhibitory function like many other members of this class of proteins.
BG tests are sometimes used in finding the reason for elevated or diminished levels of thyroid hormone. This is done by measuring resin binding to labeled thyroid hormone, which happens only when the labeled thyroid hormone is free.
The patient's serum is mixed with the labeled thyroid hormone; then, the resin is added to the whole mixture to measure the amount of free labeled thyroid hormone. So, for instance, if the patient is truly hypothyroid, and TBG levels are normal, then there are many sites open for binding on the TBG, since the total thyroid hormone level is low. Therefore, when the labeled hormone is added, it will bind mostly to the TBG, leaving little of it left for binding to the resin. In contrast, however, if the patient is truly hyperthyroid, and TBG levels are normal, the patient's endogenous hormone will saturate the TBG binding sites more, leaving less room for the labeled hormone, which allows greater binding to the resin.
In the situations described above, TBG testing is not very useful. However, if total thyroid hormone levels point to hypothyroidism or hyperthyroidism in the absence of accompanying symptoms, the utility of TBG testing becomes more evident, since TBG production can be modified by other factors such as estrogen levels, corticosteroid levels, or liver failure. If, for example, the TBG level is high, which can occur when estrogen levels are high, the TBG will bind more thyroid hormone, decreasing the free hormone available in the blood, which leads to stimulation of TSH, and the production of more thyroid hormone. In this case, the total thyroid hormone level will be high. And so, when labeled hormone is added, since TBG is so high, it will bind to the TBG, leaving little free labeled hormone for uptake into the resin. On the converse, in the presence of corticosteroids, which lower TBG levels, the total thyroid hormone (bound and free) in the blood will be low. Thus, when the labeled hormone is added, since so little TBG is available in the blood, only a small portion of it will bind, leaving plenty available for uptake by the resin.
28 вопрос
Parathyroid hormone (PTH), parathormone or parathyrin, is secreted by the chief cells of the parathyroid glands as a polypeptidecontaining 84 amino acids. It acts to increase the concentration of calcium (Ca2+) in the blood, whereas calcitonin (a hormone produced by the parafollicular cells (C cells) of the thyroid gland) acts to decrease calcium concentration. PTH acts to increase the concentration of calcium in the blood by acting upon the parathyroid hormone 1 receptor (high levels in bone and kidney) and the parathyroid hormone 2 receptor (high levels in the central nervous system, pancreas, testis, and placenta).[1] PTH half-life is approximately 4 minutes.[2] It has a molecular mass of 9.4 kDa.Regulation of serum calcium[edit]
Parathyroid hormone regulates serum calcium through its effects on the following tissues:[6]
| Region | Effect |
| bone | It enhances the release of calcium from the large reservoir contained in the bones.[7] Bone resorption is the normal destruction of bone by osteoclasts, which are indirectly stimulated by PTH. Stimulation is indirect since osteoclasts do not have a receptor for PTH; rather, PTH binds to osteoblasts, the cells responsible for creating bone. Binding stimulates osteoblasts to increase their expression of RANKL and inhibits their expression of Osteoprotegerin (OPG). OPG binds to RANKL and blocks it from interacting with RANK, a receptor for RANKL. The binding of RANKL to RANK (facilitated by the decreased amount of OPG available for binding the excess RANKL) stimulates these osteoclast precursors to fuse, forming new osteoclasts, which ultimately enhances bone resorption. |
| kidney | It enhances active reabsorption of calcium and magnesium from distal tubules and the thick ascending limb. As bone is degraded, both calcium and phosphate are released. It also decreases the reabsorption of phosphate, with a net loss in plasma phosphate concentration. When the calcium:phosphate ratio increases, more calcium is free in the circulation.[8] |
| intestinevia kidney | It enhances the absorption of calcium in the intestine by increasing the production of activated vitamin D. Vitamin D activation occurs in the kidney. PTH up-regulates 25-hydroxyvitamin D3 1-alpha-hydroxylase, the enzyme responsible for 1-alpha hydroxylation of 25-hydroxy vitamin D, converting vitamin D to its active form (1,25-dihydroxy vitamin D). This activated form of vitamin D increases the absorption of calcium (as Ca2+ ions) by the intestine via calbindin. |
 Calcium regulation in the human body.[9] The role of parathyroid hormone is shown in blue. PTH was one of the first hormones to be shown to use the G-protein, adenylyl cyclase second messenger system
Calcium regulation in the human body.[9] The role of parathyroid hormone is shown in blue. PTH was one of the first hormones to be shown to use the G-protein, adenylyl cyclase second messenger system
29Hormones insulin, glucagon, lipokain Insulin is a peptide hormone, produced by beta cells of the pancreas, and is central to regulating carbohydrate and fat metabolism in the body. It causes cells in the liver, skeletal muscles, and fat tissue to absorb glucose from the blood.
Insulin stops the use of fat as an energy source by inhibiting the release of glucagon. With the exception of the metabolic disorder diabetes mellitus and metabolic syndrome, insulin is provided within the body in a constant proportion to remove excess glucose from the blood, which otherwise would be toxic. When blood glucose levels fall below a certain level, the body begins to use stored sugar as an energy source through glycogenolysis, which breaks down the glycogen stored in the liver and muscles into glucose, which can then be utilized as an energy source. As a central metabolic control mechanism, its status is also used as a control signal to other body systems (such as amino acid uptake by body cells). In addition, it has several other anabolic effects throughout the body. l ipokain is. Iipos burn fat + kaio] - pancreatic hormone involved in the regulation of fat metabolism in the liver in the form of the drug used. the treatment of diabetes, liver disease. Glucagon, a peptide hormone secreted by the pancreas, raises blood glucose levels. Its effect is opposite that of insulin, which lowers blood glucose levels.[1] The pancreas releases glucagon when blood sugar (glucose) levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream. High blood glucose levels stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels at a stable level. Glucagon belongs to a family of several other related hormones.
30Hormones adrenaline and noradrenaline Adrenaline is a hormone produced by the adrenal glands during high stress or exciting situations. This powerful hormone is part of the human body's acute stress response system, also called the "fight or flight" response. It works by stimulating the heart rate, contracting blood vessels, and dilating air passages, all of which work to increase blood flow to the muscles and oxygen to the lungs. Additionally, it is used as a medical treatment for some potentially life-threatening conditions including anaphylactic shock. In the US, the medical community largely refers to this hormone as epinephrine, although the two terms may be used interchangeably. Noradrenaline is a neurotransmitter and a catecholamine-type hormone that is manufactured as a drug and produced naturally in the human body. Also called norepinephrine, especially by those in the medical field, this hormone acts on the parts of the brain involved with responsiveness and fear. This neurotransmitter is released into the blood from the adrenal medulla and from nerves called adrenergic nerves. As a drug, control of the noradrenaline catecholamine is commonly used to treat low blood pressure and chronic depression.
31Corticosterone, also called Kendall’s compound B, is a steroid hormone secreted by one of the outer layers of the adrenal cortex in humans. The hormone is used by the human body in response to stressors such as allergens and other environmental factors. Unlike the other steroid hormones produced by the body, corticosterone is not used for anti-inflammatory purposes. It is an antagonist for insulin use and is vital in the synthesis of carbohydrates and in protein degradation. A corticosterone molecule is composed of 21 carbon, 30 hydrogen, and four oxygen atoms.
Aldosterone is a steroid hormone (mineralocorticoid family) produced by the outer section (zona glomerulosa) of the adrenal cortex in the adrenal gland. It plays a central role in the regulation of blood pressure mainly by acting on the distal tubules and collecting ducts of the nephron, increasing reabsorption of ions and water in the kidney, to cause the conservation of sodium, secretion of potassium, increased water retention, and increased blood pressure. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and renal disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.
32Estradiol (E2 or 17β-estradiol, also oestradiol) is a sex hormone. Estradiol is abbreviated E2 as it has two hydroxyl groups in its molecular structure. Estrone has one (E1) and estriol has three (E3). Estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect. Except during the early follicular phase of the menstrual cycle, its serum levels are somewhat higher than that of estrone during the reproductive years of the human female. Thus it is the predominant estrogen during reproductive years both in terms of absolute serum levels as well as in terms of estrogenic activity. Estrone (E1, and also oestrone) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2. Estrone is an odorless, solid crystalline powder, white in color with a melting point of 254.5 °C and a specific gravity of 1.23.Estrone is one of several natural estrogens, which also include estriol and estradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily during pregnancy. Progesterone also known as P4 (pregn-4-ene-3,20-dione) is a C-21 steroid hormone involved in the female menstrual cycle, pregnancy (supports gestation) and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestogens, and is the major naturally occurring human progestogen.
33Testosterone, androsterone, methyltestorone.Testosterone is a steroid hormone from the androgen group and is found in mammals, reptiles, birds,and other vertebrates. In mammals, testosterone is secreted primarily in the testicles of males and the ovaries of females, although small amounts are also secreted by the adrenal glands. It is the principal male sex hormone and an anabolic steroid. Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone and weak androgen with a potency that is approximately 1/7th that of testosterone. In addition, it can be converted to dihydrotestosterone (DHT) from 17-hydroxyprogesterone, bypassing conventional intermediates such as androstenedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right. Methyltestosterone is a 17-alpha-alkylated anabolic steroid used to treat men with a testosterone deficiency. It bears close structural similarity to testosterone, but has a methyl group at C17 in order to increase oral bioavailability. Androsterone's 3β-isomer is epiandrosterone.
Hormones of the anterior pituitary. The chemical nature and the effect on the metabolism.
A major organ of the endocrine system, the anterior pituitary, also called the adenohypophysis, is the glandular, anterior lobe that together with the posterior lobe, the (posterior pituitary, also known as the neurohypophysis) makes up the pituitary gland (hypophysis). The anterior pituitary regulates several physiological processes including stress, growth, reproduction and lactation. Proper function of the anterior pituitary and of the organs it regulates can often be ascertained via blood tests that measure hormone levels.
Anatomy
The pituitary gland is a pea-sized gland that sits in a protective bony enclosure called the sella turcica. It is composed of three lobes: anterior, intermediate, and posterior. In many animals, these three lobes are distinct. However, in humans, the intermediate lobe is but a few cell layers thick and indistinct; as a result, it is often considered part of the anterior pituitary. In all animals, the fleshy, glandular anterior pituitary is distinct from the neural composition of the posterior pituitary.
The anterior pituitary is composed of three regions:
Pars distalis
The pars distalis, (distal part), comprises the majority of the anterior pituitary and is where the bulk of pituitary hormone production occurs.
Pars tuberalis
The pars tuberalis, (tubular part), forms a sheath extending up from the pars distalis and wrapping around the pituitary stalk. Its function is poorly understood.
Pars intermedia
The pars intermedia, (intermediate part), sits between the pars distalis and the posterior pituitary and is very small and indistinct in humans.
Physiology
The anterior pituitary contains five types of endocrine cell, responsible for endocrine secretion. They are defined by the hormones they secrete: somatotropes (GH); prolactins (PRL); gonadotropes (LH and FSH); corticotropes (ACTH) and thyrotropes (TSH).
Дата добавления: 2016-01-04; просмотров: 24; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
