Explain kinetic features of alternating copolymerization.
Copolymerization - co-polymerization of various monomers to produce polymers with improved properties,
In the reaction mixture, four elementary chain reaction reactions:

Here R1 * and R2 * are growing macroradicals ending in links M1 and M2.
The rates of consumption of monomers M1 and M2 during copolymerization are determined by the equations:
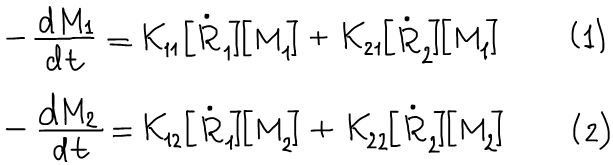
Dividing (1) by (2) we obtain:
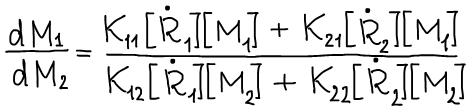
For a stationary reaction process, we can assume that the equality holds:
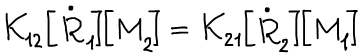
At low conversion rates, the ratio of the rates of consumption of the two monomers, or the ratio of the concentrations of the monomer units in the copolymer m1 / m2, can be described by the equation obtained by Mayo:


The copolymerization constants characterize the ratio of the rate constants of interaction of radicals with their "own" and "foreign" monomer.

3. Explain features of three-dimensional free-radicalpolymerization.
Topological features of the reaction of three-dimensional radical polymerization are observed. If one of the reagents contains two (or more) reactive C=C bonds. In this case, the copolymerization reaction of the double bonds of crosslinking agent M1 and monomer M2 branches into several topological channels
1. Growth of linear chains carrying side double bonds and terminal radicals

2. primary intramolecular cyclization

3. intermolecular cross-linking of chains

Processes of TFRP for polyunsaturated oligomers (monomers) of various structures have a similar development pattern. At the initial stage (in nonstructured reaction medium), the polymerization rate W0 remains constant in a rather narrow range of conversions, 0 < C < 3 − 4%; then, in the period of polymerization auto-acceleration, it increases with growing C, and then with conversion Cmax, it becomes maximal Wmax.
Kinetic features: W0 increases with conversions C → 0 as the viscosity grows. In its turn, reaction medium viscosity for small conversions is regulated by intensity of intermolecular interactions (IMI) of molecules of initial oligomers. Dependence W0 = f(η) is sufficiently universal for oligo(acrylates) polymerization and trivial in terms of its physical sense. Indeed, W0 is a function of the following kinetic parameters: 
where Wi = initiation rate; kpr and kter = constants of chain propagation rate and chain termination rate; and [M] = oligo(acrylates) concentration.
Dependence of initial polymerization rate W0 on oligo(acrylates) nature comprises not only viscosity influence. In some cases at the initial TFRP stage, abnormal reactivity of oligomers was observed, and this reactivity was interpreted based on the assumption implying the existence of regular kinetically active associates in liquid oligo(acrylates). For example, when measuring values of chain propagation rate constant kpr and chain termination rate constant kter during polymerization of (alkylene glycol) dimethyl acrylates with different sizes of hydrocarbon chain ( CH2 )n for conversions C → 0, an abnormally high value of kpr was found, which increased threefold as n increased from 4 to 10.
Дата добавления: 2018-02-15; просмотров: 641; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
