Text D. Conventional Water Treatment.
Disinfection. Additional Treatment
Disinfection is the complex of measures for destroying agents of in- fection in the water with the help of various disinfectants. It is accom- plished both by filtering out harmful microorganisms and by adding dis- infectant chemicals for killing any pathogens which pass through the filters.
There are several methods of treatment of water to kill living organ- isms, particularly pathogenic bacteria; chlorination (the application of chlorine or chlorine compounds – chloramine and chlorine dioxide) is the most common. Chlorine is a strong oxidant and a toxic gas. Chlorine dioxide has more recently been found effective as a destroyer of bacteria, as well as a means of removing undesirable tastes and odours. Chlorine has limited effectiveness against protozoans that form cysts in the water.
Less frequently used methods include the use of ozone, ultraviolet light, or silver ions. Boiling is the favored household emergency meas- ure.
The advantage of ozonation over chlorination is the production of fewer dangerous by-products and the absence of taste and odour. Ozone gas is a colourless toxic gas with powerful oxidizing properties, formed from oxygen by electrical discharges or ultraviolet light. It is an effective method to destroy harmful protozoans that form cysts in the water and to kill almost all other pathogens. Ozone is a very strong, broad spectrum disinfectant widely used in Europe.
UV radiation (light) is very effective against inactivating cysts.
The main disadvantage of ozonation and UV radiation is that they leave no disinfectant residual in the water, and it is sometimes necessary to add a residual disinfectant afterwards.
69

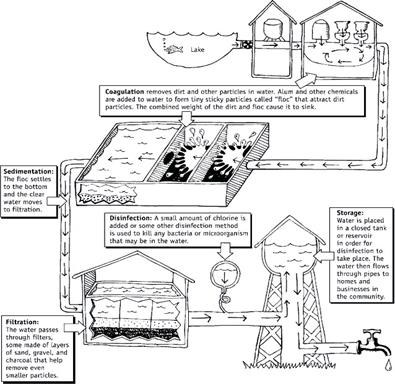
Pict. 11. Water from the Source through the Water Treatment Process
Some additional treatment methods include:
1. softening (the process of removing the dissolved calcium and magnesium salts that cause hardness in water, either by adding chemicals or by ion exchange);
2. aeration (the process of spraying water into the air used for taste and odour control and for removal of dissolved iron and manganese);
3. (activated) carbon adsorption (the process of adsorption impuri- ties by activated carbon (saturation carbon with impurities) used for re- moving dissolved organic substances that cause tastes, odours, or col- ours);
70
4.  distillation (the separation of dissolved solids from water by evap- oration and condensation);
distillation (the separation of dissolved solids from water by evap- oration and condensation);
5. deferrization (the removal of iron from water);
6. desalination (desalinization) (any of several processes that remove excess salt and other minerals from water);
7. fluoridation (the addition of sodium fluoride or other fluorine compounds to filtered water for reducing tooth decay);
8. reverse osmosis (a process by which water passes through a porous membrane which passes the water, but does not pass the impurities dis- solved in it).
Water treatment plants employ a variety of treatment methods. These processes are used in varying combinations, depending on the character- istics of water and on its intended use.
 |
18. Read the texts of Unit 3 again and make notes under the follow- ing headings. Then use your notes to talk about Water Quality, Water Pollution and Water Treatment and Conventional Water Treatment.
1. The interconnection among water quality, water pollution and water treatment.
2. Water composition. Types of water impurities.
3. Conventional water treatment.
71




 Pict. 12. Drinking Water Treatment: through the Ages
Pict. 12. Drinking Water Treatment: through the Ages
72


Unit 4
Дата добавления: 2019-02-22; просмотров: 266; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
