Carbon Monooxide ( charcoal gas).
F orms at interaction of CO2 with an incandescent coal:
CO2 + C Û CO DHо = 171,5 kJ/mol
The reaction is reversible, its shift to the right is predetermined by an entropy factor, and to the left – by enthalpy. Below 400° reaction is practically fully displaced to the left, and higher than 1000°, to the right. At ordinary conditions, CO is fully stable substance.
Preparation.
In industry: in great scale - in the form of generator and aquatic gases.
Generator gas forms at incomplete combustion of anthracite coal or `coke in large furnaces (generators). It is the mixture of gases: 25% CO, 70% H2, 4% CO2, other - N2, CH4, O2.
Water-gas forms at passing of water steam above incandescent carbon:
С + Н2О = СО + Н2 DН°298 = 117,6 kJ/mol
In a laboratory: at decomposition of formic or oxalic acid or their salts at intearaction with hot concentrated H2SO4:
НСООН = СО + Н2О
Н2С2О4 = СО + СО2 + Н2О
Structure. A molecule CO is extraordinarily stable (one of most stable among the known molecules). It sustains heating to 6000°. Energy of bond (1069 kJ/mol) is more than in H2 (938 kJ/mol) and to the triple bond between atoms:
 |
 C O
C O
 |
 2s 2p 2s 2p
2s 2p 2s 2p
 |



 C O or C O
C O or C O
In accordance with a method of Valence bonds two bonds are formed at coupling of two unpaired electrons of carbon and two - of O and both are displaced toward oxygen; another bond forms according donor-acceptor mechanism: to two-electronic orbital of oxygen is overlapped with a lone electron orbital of carbon. This pair is displaced to carbon, and on the whole the molecule of CO is weakly polar (m = 0,37•10-29 C.m).
Properties. Small polarity and capacity for polarizing predetermine low b.p. -191,5° and low solubility in water (3,3 vol. at 100 vol. H2O).
 CO is an extraordinarily toxic gas. Its content in air must not exceed 0,02 mg/l. At slight poisoning, fresh air is an antidote. Qualitatively, the presence of CO in air can be revealed by passing it through a solution of PdCl2, which darkens:
CO is an extraordinarily toxic gas. Its content in air must not exceed 0,02 mg/l. At slight poisoning, fresh air is an antidote. Qualitatively, the presence of CO in air can be revealed by passing it through a solution of PdCl2, which darkens:
CO + PdCl2 + H2O = Pd¯ + CO2 + 2HCl
At ordinary conditions, CO is nonsaltforming oxide, it is unreactive with water, acids and alkalis. With alkalis, it reacts only at elevated temperatures, and at the presence of catalysts:
СО + NaOH = HCOONa,
So, CO acts as the anhydride of formic acid.
For CO there are characteristic reactions of addition and oxidation, where it is reduced:
Fe2O3 + 3CO = 2Fe + 3CO2
NiO + CO = Ni + CO2
CO + Cl2 = COCl2 (phosgene)
COCl2 is slowly decomposed by water, quicker by alkalis and it is a typical chloroanhydride:
COCl2 + H2O = CO2 + 2HCl
Phosgene is very poisonous substance and was used as a chemical weapon.
CO finds wide application in the organic syntheses. It is used as a reagent for preparation of paraffins, petrol, methyl alcohol, aldehydes, a lot of acids (formic, oxalic, vinegar, propionic, adipinic, acrylic), hydrogen cyanide and many other products.
At higher pressures CO reacts with powdered metals with formation of complex metal carbonyls: Fe(CO)5, Co2(CO)8, Ni(CO)4, Cr(CO)6 and others. Ligand in these compounds uses unshared electronic pair of carbon. Carbonyls are easily decomposed; they find application for preparation of pure metals. Like CO, all of them are very poisonous.
OXYGEN CONTAINING COMPOUNDS
Silicon
Silicon dioxide. Si interacts with O vigorously when heated:
Si + O2 = SiO2 DH°298 = -908 kJ / mol
SiO2 structure. Natural SiO2 occurs in crystalline (quartz) and amorphous forms. Crystalline SiO2 exists as a set of several polymorphs, which are mutually transformed from one into another in accordance with the following scheme:
 β-quartz 870 º β-tridymite 1470 º β-cristobalite 1723º liquid
β-quartz 870 º β-tridymite 1470 º β-cristobalite 1723º liquid
575 º 130 º 270 º <1650 º
α-quartz α-tridymite α-cristobalite glass
Quartz, tridymite, and crystobalite can have mutual transitions from one to another, but they are very slow. Consequently, tridymite, and crystobalite can be stored very long time at room temperature and exist as separate minerals despite their thermodynamic instability. Note that a -modifications are stable at room temperature and b -modifications — at high temperature. At the rapid cooling of the melt, crystallization has not time to occur and amorphous vitreous form, fused silica (quartz glass) appears.
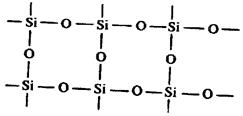
Various modifications of SiO2 are inorganic polymers with heterochains.
|
|
These giant molecular entities have strong covalent bonds Si—O—Si. Each Si atom is surrounded by tetrahedron of four O atoms and O atoms, in turn, connect to each other two such tetrahedrons.
These connected tetrahedrons twisted in a spiral like staircase of the type of screw. Spirals have parallel orientation in space. The crystalline form with rightscrew spirals is called a -quartz, and with leftscrew spirals is called b -quartz.
Properties. Polymeric SiO2 is not soluble in water at STP. It is stable to attacks of acids. It dissolves only in hydrofluoric acid, HF, due to the formation of volatile SiF4 with stronger Si—F than Si–O bonds:
SiO2 + 4HF = SiF4 + 2H2O
SiO2 dissolves slowly in alkali solutions, and quickly in their melts forming silicates:
SiO2 + 2NaOH = Na2SiO3 + H2O
SiO2 is a silicic acid anhydride. As a compound with extremely low volatility (fugitivity), it displaces other acid anhydrides when melting with salts that are used in glassmaking:
SiO2 + Na2SO4  Na2SiO3 + SO3
Na2SiO3 + SO3
Silicic acids. The overwhelming majority of meta-silicic acid, H2SiO3, salts are insoluble in water. Na2SiO3 is one of the few soluble salts. It is called “soluble glass” and aqueous solutions of the salt – “liquid glass”.
H2SiO3 is a very weak acid. Its soluble in water fraction of molecules dissociates in negligible small degree (K1 = 1 ∙ 10-10). Liquid glass forms strong alkaline medium and high viscosity after hydrolysis due to the formation of polymorphs of orthosilicic acid (silicate glue):
Na2SiO3 + H2O = Na2H2SiO4
Na2H2SiO4 + 2H2O = H4SiO4 + 2NaOH
Some salt H2SiO3 with weak bases completely hydrolyze in a solution. Therefore, they cannot be obtained by the double replacement reaction:
Na2SiO3 + 2NH4Cl + H2O = H4SiO4 + NaCl + 2NH3
Metasilicic acid is displaced from its salts in aqueous solutions by other acids, including H2CO3. At first, water-soluble orthosilicic acid appears which can exist in very dilute solutions:
Na2H2SiO4 + 2HCl = H4SiO4 + 2NaCl
|
|
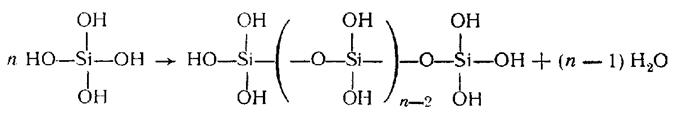
The growth of concentration or just standing leads to polymerization:
The elementary block of such a chain polymer is H2SiO3, but the further condensation leads to branched, cross-linked, bulk polymers, and, eventually, polymeric SiO2 due to transformations of OH-groups:
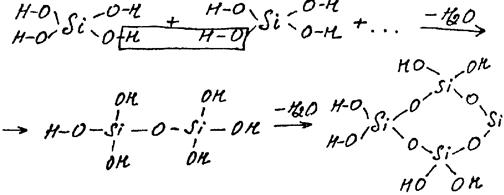
 H – O O – H H - O O – H
H – O O – H H - O O – H
Si + Si + -H2O
 H – O O – H H – O O – H . . .
H – O O – H H – O O – H . . .
 HO OH
HO OH
Si
OH OH H - O O O
H – O – Si – O – Si – OH -H2O Si Si

OH OH H – O O O
Si
HO OH
Tetrahedral surrounding of silicon by oxygen atoms occurs in any polymeric form of silicic acid. These polymers are insoluble and have nonstoichiometric composition (SiO2)x∙(H2O)y. When x> 1 they correspond to polysilicic acids, which derivatives is a variety of natural silicates.
Silicate materials are produced artificially in large quantities. The maximal amount among synthetic silicates has glass, which composition expresses the formula Na2CaSi6O14 or Na2O∙CaO∙6SiO2. The partial replacement of Na, Ca, Si atoms by the atoms of other elements gives a great number of different kinds of special glass depending on the nature of its application. For example, laboratory glassware contains significant content of B2O3 and Al2O3. It is characterized by high chemical stability (especially, in acidic medium) and low coefficient of thermal incandescences.
Silicon halides. Silicon forms halides of general formula SiX4 with halogens:
SiO2 + 2C + 2Cl2  SiCl4 + 2CO
SiCl4 + 2CO
Tetrahalides are very reactive substances. They hydrolyze easily as halogenanhydrides of ortho-silicic acid:
SiCl4(aq) + 4H2O = H4SiO4(aq) + 4HCl(aq) DG°298 = -238 kJ / mol
Hydrolysis is a result of consecutive H2O molecules addition and HX molecules removal up to H4SiO4 formation.
SiX4 fumes in air due to hydrolysis (e.g. SiCl4 is used to create artificial fog). SiCl4 is an active chlorinated agent that interacts with oxides of metals and nonmetals:
3 SiCl4 + 2Al2O3 = 3SiO2 + 4AlCl3
SiF4 interacts with HF acid forming hexafluorosilicic acid, H2SiF6:
SiF4 + 2HF = H2[SiF6]
Si atom in H2SiF6 is in sp3d2-hybrid state and its coordination number is 6. H2SiF6 is not known in the free state, it is a strong acid (» H2SO4).
Other silicon tetrahalides do not form hexahalido compounds. Their resistance to various reagents and heating decreases toward SiI4.
Silicon nitride. Amorphous Si interacts with N2 only at t>1300 °C. Silicon nitride, Si3N4, formed is a solid stable compound that only decomposes slowly by molten alkali or hot concentrated HF:
Si3N4 + 12NaOH = 3Na2SiO3 + 4NH3
Si3N4 + 16HF = 2(NH4)2SiF6 + SiF4
Silicon carbide. Si or SiO2 react with carbon in electric furnaces at t » 2300 °C. SiC is a very hard (the second after diamond) refractory material. It is chemically stable at normal conditions, but decomposes when heated in molten alkali (in the presence of O2), Cl2, hot steam:
SiC + 4NaOH + 2O2 = Na2SiO3 + Na2CO3 + 2H2O
¯8e 2e×2
SiC + 2Cl2  SiCl4 + C
SiCl4 + C
SiC + 2H2O 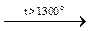 SiO2 + CH4
SiO2 + CH4
CHEMICAL COMPOUNDS
Compounds with oxygen
Germanium, tin, and lead
Compounds +2. There are oxides (GeO, SnO - black, PbO - yellow-red (litharge), hydroxides M(OH)2 - white. Salts of oxygencontaining acids are not typical for Sn2+ (and, especially, Ge2+). However, SnSO4 is used in electrolytic tinning[1].
EO oxides are produced by hydroxides decomposition when heated in a stream of N2:
Е(OH)2 = ЕO + H2O (Е - Ge, Sn)
PbO:
2Pb(NO3)2 = 2PbO + 4NO2 + O2
2Pb + O2 = 2PbO
EO are insoluble in water, so E(OH)2 are obtained by the double replacement reactions of soluble salts with alkalis. EO and E(OH)2 are amphoteric since dissolve in acids and alkalis:
Sn(OH)2 + 2HCl = SnCl2 + 2H2O
Sn(OH)2 + 2NaOH = Na2[Sn(OH)4]
In the series Ge — Sn—Pb basic properties of oxygen-containing compounds are enhanced, and acid properties are reduced: in case of Ge(OH)2 predominate acidic, and in Pb(OH)2 basic properties. For instance, Pb(OH)2 has K1basic = 10-5, and K1acid = 10-11.
Hydrolysis. Soluble E2+ salts hydrolyse in water. In connection with the strengthening of basic properties of hydroxides in the series Ge(OH)2 – Sn(OH)2 – Pb(OH)2degree ofhydrolysis of salts decreases. The lowest hydrolysis degree is observed in case of soluble salts of Pb: for example, Pb(NO3)3 gives acid reaction. Ge2+ salts almost completely decomposed by water in dilute solutions with the formation of Ge(OH)2. Salts of Sn (II) occupy an intermediate position:
SnCl2 + H2O Û SnOHCl¯ + HCl
HCl solution is added to avoid formation of basic salt precipitate.
Insoluble in water compounds are: PbSO4, PbCrO4, PbBr2, PbI2, with low solubility - PbCl2. The soluble salts are nitrate, acetate, hexafluorosilicate. Pb(CH3COO)2 has a sweet taste and is called lead sugar. All salts of lead are poisonous.
Red-Ox. Oxidation state (+2) is an intermediate state between typical for these elements 0 and (+4), so they can both oxidants and reductants. Oxidising properties are expressed very poorly, (Ео(Ge2+/Ge) = 0,2 V, Ео(Sn2+/Sn) = -0,136 V, Ео(Pb2+/Pb) = -0,126 V, which are close to 0). Examples of these E2+ properties are given below:
PbO + CO = Pb + CO2, or
SnCl2 + Zn = Sn + ZnCl2
Pb(CH3COO)2 + Fe = Pb + Fe(CH3COO)2
Reducing properties in the series Ge—Sn—Pb diminish. They are expressed more strongly in alkaline medium than in acidic (compare the standard potentials):
Е°Sn4+/Sn2+ = + 0,15 V; E° Sn(OH)6-2/Sn(OH)4-2 = -0,9 V
Е°Pb4+/Pb2+ (H+)= + 1,68 V; E° PbO2/Pb(OH)2(OH-)= +0,28 V
SnCl2 is often used in practice of chemists as a chemical reductant:
SnCl2 + 2FeCl3  2FeCl2 + SnCl4
2FeCl2 + SnCl4
SnCl2 + HgCl2  Hg + SnCl4
Hg + SnCl4
3SnCl2 + 2BiCl3 + 12NaOH = 2Bi¯ + 3Sn(OH)4¯ + 12NaCl
3GeCl2 + 2K2Cr2O7 + 16HCl = 3GeCl4 + 2CrCl3 + 4KCl + 8H2O
Pb2+ is a very weak reductant. Therefore, it can be oxidised only by the strongest oxidants in alkaline medium:
Pb(OH)2 + Cl2 + 2NaOH = PbO2 + 2NaCl + 2H2O
Na2[Pb(OH)4] + Br2 + 2NaOH = PbO2 + 2NaBr + 2H2O
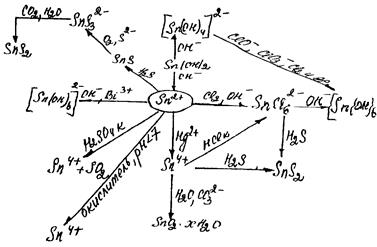
Compounds (+4). Production. GeO2 and SnO2 are produced by the direct combination of elements. PbO2 is obtained in the laboratory by Pb(CH3COO)2 oxidation with chlorinated lime:
Pb(CH3COO)2 + CaOCl2 + H2O = PbO2¯ + CaCl2 + 2CH3COOH
Properties. GeO2 is an acid oxide. Unlike SiO2, SnO2, and PbO2, it is slightly soluble in water (4 g/l), forming hydrates of varying composition, called germanic acid. Meta-H2GeO3 and ortho-H2[Ge(OH)6] are known. This is a weak acid:
H2GeO3 Û H+ + HGeO3- K1 = 1.10-9
HGeO3- Û H+ + GeO32- K2 = 2.10-13
However, GeO2 displays amphoteric properties (reacts with alkalis, forming hydroxogermanates)
GeO2 + NaOH + 2H2O = Na2[Ge(OH)6],
and when heated with concentrated HCl it forms GeCl4:
GeO2 + 4HCl = GeCl4 + H2O
SnO2 is a polymeric inactive substance. It does not react with acids and alkali solutions, but reveals amphoteric properties (when heating, Н2SO4(conc.) or fusion with alkalis):
SnO2 + 2Н2SO4(conc.) = Sn(SO4)2 + 2H2O
SnO2 + 2NaOH = Na2SnO3 + H2O
Tin (+4) hydroxide can be prepared by the double replacement reaction of soluble compounds:
SnCl4 + 4NaOH = Sn(OH)4¯ + 4H2O
This white amorphous precipitate is a polymer of varying composition (SnO2) х ∙( Н 2 О )y. Some simplified formulae can be offered:
H2SnO3 or SnO(OH)2 (x = 1, y = 1);
H4SnO4 оr Sn(OH)4 - ortho-form (x = 1, y = 2).
Freshly prepared precipitate is called a -stannic acid. It has amphoteric nature and reacts with alkalis and acids easily:
Sn(OH)4 4HCl = SnCl4 + 4H2O
Sn(OH)4 + 2NaOH = Na2[Sn(OH)6]
When standing (sooner when heating), the process of Sn(OH)4 “aging” takes place, transforming into inactive form - b-stannic acid. The process of aging illustrates the following scheme:
PbO2. There are two polymorphs: orthorhombic a -PbO2 and tetragonal b -PbO2. b-PbO2 is the most stable at normal conditions. b-PbO2 ® a-PbO2 transition occurs at 300 oC and pressure > 13 000 atm. At atmospheric pressure b-PbO2 decomposes at 280 oC (a-PbO2 at 220 oC):
3PbO2 = Pb3O4 + O2
Preparation.
1. Action of HNO3 on Pb3O4:
Pb3O4 + 4HNO3 = Pb(NO3)2 + PbO2 + 2H2O not H4PbO4
2. Anode oxidation of Pb2+ salts:
PbSO4 + 2H2O -2e ® PbO2 + H2SO4 + 2H+
3. the action of strong oxidants on them:
Pb(CH3COO)2 + Cl2 + 2H2O = PbO2 + 2CH3COOH + 2HCl
Properties. PbO2 is insoluble and does not react with water. This is an amphoteric oxide and, like SnO2, it reacts with acids and alkalis at rigid conditions. The difference is that the salts formed, for example, Na2PbO3 or Pb(SO4)2 do not exist in aqueous solutions and hydrolyse completely. PbO2 is not soluble in dilute H С l, H2SO4 , HNO3 , but it dissolves in a mixture of diluted HNO3 + H2O2 , and concentrated H С l , Н 2 SO4 :
PbO2 + 2HNO3 + H2O2 = Pb(NO3)2 + O2 + 2H2O
PbO2 + 4HCl(conc.) = PbCl2 + Cl2 + 2H2O (like MnO2)
2PbO2 + 2H2SO4(conc.) = PbSO4 + O2 + 2H2O
Fine precipitate of PbO2 is soluble in alkali solutions:
PbO2 + 2NaOH + 2H2O ® Na2[Pb(OH)6]
PbO2 is a very strong oxidant (in acidic medium):
5PbO2 + 2MnSO4 + 3H2SO4 = 5PbSO4 + 2HMnO4 + 2H2O
PbO2 + Cr3+ + H2O 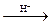 Cr2O72- + 3Pb2+ + 4H+
Cr2O72- + 3Pb2+ + 4H+
Hydrated PbO2 form does not exist (they are extremely unstable), i.e. Pb(OH)4 or plumbic acids are not known. However, salts of these acids (plumbates) can be obtained for many metals, including Pb2+:
PbO + PbO2 = PbPbO3 (Pb2O3) Pb(II) meta-plumbate
2PbO + PbO2 = Pb2PbO4 (Pb3O4) Pb(II) ortho-plumbate
Pb3O4 is called red lead and used as a red-orange pigment. It can be prepared by heating PbO in air:

Pb3O4 reacts with strong acids; its oxidation state does not change, confirming that Pb3O4 can be attributed to salts:
Pb2PbO4 + 4HNO3 = 2Pb(NO3)2 + H4PbO4 ® PbO2 + 2H2O (immediate decomposition)
Pb3O4 dissolves in alkalis:
Pb2PbO4 + 6NaOH + 4H2O = 2Na2[Pb(OH)4] + Na2[Pb(OH)6]
This is a strong oxidant:
Pb3O4 + 8HCl = 3PbCl2 + Cl2 + 4H2O
In summary, Ge subgroup oxides stability can be described by the scheme:
oxidizing properties growth
 GeO2 SnO2 PbO2
GeO2 SnO2 PbO2
stability growth
oxidation reduction
GeO SnO PbO
reducing properties growth
Thus, there is an increase of the lowest oxidation state stability in accordance with amplification of metallic properties in the series Ge—Sn—Pb.
Дата добавления: 2022-01-22; просмотров: 26; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!