Oxides and hydroxides E(III).
Production. In industry: 2Е2S3 + 3O2 = 2E2O3 + 6SO2
In laboratory: 4Е + 3О2 = 2Е2О3
2Sb + 2HNO3(dil.) = Sb2O3 + 2NO + H2O
4Bi(NO3)3 = 2Bi2O3 + 12NO2 + 3O2
Structure. Like Р2О3, As2O3 forms dimer As4O6 molecules with a tetrahedral structure in the gaseous state. As2O3 forms three crystalline polymorphs and a glassy one in the solid state. Sb2O3 oxide forms two crystalline polymorphs. Pseudomolecular Sb2O3 structure, which consists of chains —Sb—O—Sb—O—Sb— and oxygen bridges between them, is stable at STP:
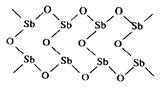
Unlike these nitric oxide Bi2O3 focal forms tetragonal lattice structure, so it is more refractory and less volatile.
Properties. Basic properties of oxides are increased when the radius of an atom becomes larger:

Chemical properties of these oxides illustrate reactions with water, acids and alkalis. First of all, let us recall:
N2O3 + H2O = 2HNO2
P2O3 + 3H2O = 2H3PO3 (phosphorous acid, dibasic)
N2O3 + 2NaOH = 2NaNO2 + H2O
These oxides belong to typical acidic nature oxides.
As2O3 is soluble in water:
As2O3 + H2O 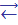 2HAsO2 meta-arsenous acid
2HAsO2 meta-arsenous acid
This is a very weak acid (K = 7.10-10). It also reveals much weaker basic properties:
OAsOH 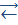 OAs+ + OH- К = 5.10-15
OAs+ + OH- К = 5.10-15
illustrating the growth of basic properties in the series As—Sb—Bi.
A meta-arsenous acid can combine water:
HAsO2 + Н2О 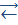 H3AsO3 ortho-arsenous
H3AsO3 ortho-arsenous
The equilibrium of this process is strongly shifted to the left, i.e. meta-form predominates.
As2O3 reacts with alkalis:
As2O3 + 2NaOH  2NaAsO2 (meta-) arsenite
2NaAsO2 (meta-) arsenite
As2O3 unlike Р2О5 also interacts with HHal acids:
As2O3 + 8HCl = 2HAsCl4 + 3H2O
Sb2O3 is not soluble in water, but reacts with acids:
Sb2O3 + 3H2SO4 = Sb2(SO4)3 + 3H2O
and concentrated alkalis:
Sb2O3 + 2NaOH + 3H2O = 2Na[Sb(OH)4] meta-stibnite
(antymonite, stibnite)
Sb2O3 + 6NaOH + 3H2O = 2Na3[Sb(OH)6]
(ortho-stibnite, antymonite)
Bi2O3 is not soluble in water, has no interaction with alkalis, but dissolves in acids:
Bi2O3 + 6HCl = 2BiCl3 + 3H2O
Properties of hydroxides changed in a similar way: all of them are amphoteric, acid character predominates in As(OH)3 basic properties – in case of Sb(OH)3, and acid behaviour of Bi(OH)3 is expressed so poorly that it only appears as a very low solubility of this hydroxide in concentrated solutions of strong alkalis. Therefore, the acid character of hydroxides E(OH)3 is rapidly weakening in the series As—Sb—Bi:
|
|
|

 HNO3 H3PO3 H3Al3O3 Sb(OH)3 Bi(OH)3
HNO3 H3PO3 H3Al3O3 Sb(OH)3 Bi(OH)3

Acids amphoteric bases
mainly acid mainly base
Consider:
acid HNO2 + NaOH = NaNO2 + H2O
H3PO3 + NaOH = Na2HPO3 + H2O
Amphoteric H3AsO3 + 3NaOH = Na3AsO3 + 3H2O
H3AsO3 + 3HCl = AsCl3 + 3H2O
Sb(OH)3 + 3HCl = SbCl3 + 3H2O
Sb(OH)3 + 3NaOH = Na3[Sb(OH)6]
Basic Ві(ОН)3 + NaOH ® don’t react
Dissolved hydroxides of Sb and Bi can dissociate simultaneously by both mechanisms:
E3+ + 3ОН- 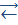 E(ОН)3 º Н3EО3
E(ОН)3 º Н3EО3 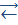 3Н+ + EО33-
3Н+ + EО33-
Ortho-arsenous H3AsO3 acid known only in a solution is very weak:
H3AsO3 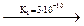 H+ + H2AsO3-
H+ + H2AsO3- 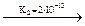 H+ + HAsO32-
H+ + HAsO32- 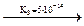 H+ + AsO33-
H+ + AsO33-
Sb(OH)3 is also stable in a solution only. Acid properties of Sb(OH)3 are much weaker than H3AsO3 (K1 = 10-11), but there are complex ions: [Sb(OH)4]-— metastibite [Sb(OH)6]3-—orthostibite in strong alkali solutions.
The white flaky precipitate is formed at interaction of Bi3+ salts with alkali:
Bi(NO3)3 + 3NaOH = Ві(ОН)3¯ + 3NaNO3
Ві(ОН)3 is not soluble in excess of alkali (acid properties are not typical).
Since basic properties of hydroxides Е ( ОН )3 in the series As—Sb—Bi grow, stability of salts with E3+ cation grows in the same series similarly: such salts of As3+ are not isolated (for oxygen-containing acids), few examples of Sb3+ salts are known (sulfate) and in case of Bi it is a typical feature of the element.
|
|
|
Soluble Sb3+ and Ві3+ salts are known in strongly acidic solutions only. They hydrolyse after dilution with the formation of basic salts:
(Ві) SbCl3 + H2O 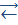 Sb(OH)Cl2 + HCl
Sb(OH)Cl2 + HCl
(Ві) Sb(OH)Cl2 + H2O 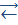 Sb(OH)2Cl + HCl
Sb(OH)2Cl + HCl
Hydrolysis products can lose water molecules forming oxo-ions:
Sb(OH)2Cl 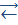 SbOCl + Н2О
SbOCl + Н2О
SbO+ — antymonil, BiO+ — bismuthil.
The higher basicity of corresponding hydroxide, the lower its hydrolysis degree. On the other hand, AsCl3, like PCl3, is not a salt, its hydrolysis is irreversible and two acids are the products of the reaction:
AsCl3 + 3H2O = H3AsO3 + 3HCl (AsCl3 is a halogenanhydride)
In the series As3+—Sb3+—Bi3+ reducing properties are decreased (the tendency of oxidation to higher valence) together with the growth of basic properties of E(OH)3 and weakened acid ones as a result of increased stability of compounds:
Na2HPO3 + I2 + 3NaOH = Na3PO4 + 2NaI +2H2O
Na3AsO3 + I2 + 2NaOH = Na3AsO4 + 2NaI + H2O
Na3[Sb(OH)6] + Br2 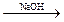 Na[Sb(OH)6] + 2NaBr
Na[Sb(OH)6] + 2NaBr
Bi(OH)3 + Cl2 + 2KOH = KBiO3 + 2KCl + H2O
Compounds E (5+). As2O5 (arsenic anhydride) and Sb2O5 can be obtained through careful heating of their hydrates (obtained by oxidation of As and Sb in concentrated HNO3):
2H3AsO4 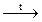 As2O5 + 3H2O
As2O5 + 3H2O
2HSbO3 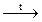 Sb2O5 + H2O
Sb2O5 + H2O
Bismuth oxide, Ві2O5, can be obtained by the reaction:
Ві2O3 + О3 (О.О2) = Ві2O5 + 2О2
¯2е. 2е
Stability of these oxides decreases in the series As—Sb—Bi, so decomposition temperature also decreases:
As2O5 (Sb2O5) 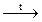 As2O3 + O2 t ~ 500oC
As2O3 + O2 t ~ 500oC
Bi2O5 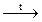 Bi2O3 + O2 t ~ 100oC
Bi2O3 + O2 t ~ 100oC
Arsenic acid, H3AsO4, which corresponds to As2O5, can be obtained
As2O5 + 3H2O = 2H3AsO4
or by As oxidation with strong oxidants:
3As + 5HNO3 + 2H2O = 3H3AsO4 + 5NO
2As + 5NaClO + 3H2O = 2H3AsO4 + 5NaCl
2As + 5Cl2 + 8H2O = 2H3AsO4 + 10HCl
H3AsO4 is easily dissolved in H2O and close to H3PO4 (K1 = 6.10-3, K2 = 1.10-7, K = 3.10-12)
Sb2O5 is poorly soluble in water, it reacts much better with alkalis:
Sb2O5 + 2KOH + 5H2O = 2K[Sb(OH)6].
The corresponding acid is not isolated in a free state. An effort to get antimonic acid usually results in the precipitate of nonstoichiometric compound, Sb2O5∙nH2O. Acid properties of antimonic acid are expressed very weakly (К1 = 4.10-5).
|
|
|
Дата добавления: 2021-03-18; просмотров: 47; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
