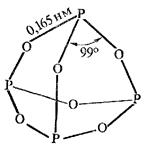
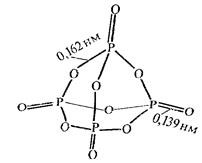
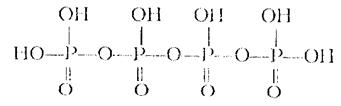Tetrametaphosphoric 2. Tetrapolyphosphoric
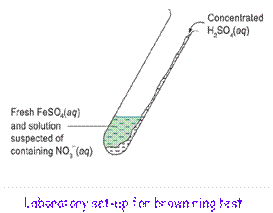
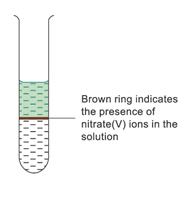
The brown ring test is used to detect nitrate(V) ions in aqueous solutions
Procedure:
1. Mix a freshly prepared FeSO4 solution with a solution suspected of containing nitrate(V) ions in a test tube.
2. Concentrated H2SO4 is added carefully along the side to the bottom of the test tube with the test tube tilted.
3. Formation of a brown ring confirms the presence of nitrate(V) ions in the solution.
Oxides and oxoacids of phosphorus
|
|
Phosphorus(III) oxide , P4O6 (also called tetraphosphorus hexoxide) and phosphorus(IV) oxide, P4O10 (or tetraphosphorus decoxide) are acid anhydrides of phosphorus oxyacids and hence readily react with water.
P4O6 + 6 HCl → 2 H3PO3 + 2 PCl3
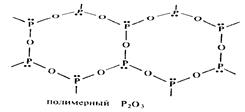 P4O10 is a particularly good dehydrating agent that can even remove water from nitric acid, HNO3. The structure of P4O6 is like that of P4 with an oxygen atom inserted between each of the P-P bonds.
P4O10 is a particularly good dehydrating agent that can even remove water from nitric acid, HNO3. The structure of P4O6 is like that of P4 with an oxygen atom inserted between each of the P-P bonds.
|
|
The structure of P4O10 is similar to that of P4O6 with the addition of one oxygen bond to each phosphorus atom via a double bond and protruding away from the tetrahedral structure.
The desiccating power of P4O10 is strong enough to convert many mineral acids to their anhydrides.
Examples: HNO3 is converted to N2O5; H2SO4 is converted to SO3; HClO4 is converted to Cl2O7.
Oxoacids of phosphorus
| Oxidation State | Formula | Name | Acidic Protons | Compounds |
| +1 | H3PO2 | hypophosphorous acid | 1 | acid, salts |
| +3 | H3PO3 | (ortho)phosphorous acid | 2 | acid, salts |
| +5 | (HPO3)n | metaphosphoric acids | N | salts (n=3,4) |
| +5 | H5P3O10 | triphosphoric acid | 3 | salts |
| +5 | H4P2O7 | pyrophosphoric acid | 4 | acid, salts |
| +5 | H3PO4 | (ortho)phosphoric acid | 3 | acid, salts |
 Hypophosphorous acid is a phosphorus oxoacid and a powerful reducing agent with molecular formula H3PO2. HOP(O)H2 exists in equilibrium with the minor tautomer HP(OH)2. Sometimes the minor tautomer is called hypophosphorous acid and the major tautomer is called phosphinic acid.
Hypophosphorous acid is a phosphorus oxoacid and a powerful reducing agent with molecular formula H3PO2. HOP(O)H2 exists in equilibrium with the minor tautomer HP(OH)2. Sometimes the minor tautomer is called hypophosphorous acid and the major tautomer is called phosphinic acid.
|
|
|
8Р + 3Ва(ОН)2 + 6Н2О = 2РН3 + 3Ва(Н2РО2)2
PH3 + 2I2 + 2H2O → H3PO2 + 4I− + 4H+
Ва(Н2РО2)2 + H2SO4 = 2Н3РО2 + BaSO4
sp3-is a hybrid state of Р due to p-bonding with О.
It is relatively strong monobasic acid :
Н3РО2 = Н+ + Н2РО2- k = 8.5.10-2
Anhydride of this acid is not known.
Hypophosphites are well soluble in water and have strong reducing properties:
NiCl2 + 2 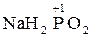 + 2H2O = Ni¯ + 2
+ 2H2O = Ni¯ + 2 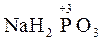 (phosphite) + 2H2
(phosphite) + 2H2
2е ¯2е 1е.2
Н3РО2 can but rarely be also an oxidant:
Н3РО2 + 2Zn + 4HCl [H] = 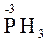 + 2ZnCl2 + 2H2O
+ 2ZnCl2 + 2H2O
Hypophosphorous acid can also disproportionate when heated:
|
|
3 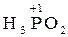 =
= 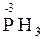 + 2
+ 2 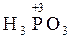
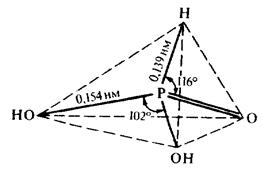
Phosphorous acid.
Preparation:
РX3 + 2Н2О = Н3РО3 + 3НX
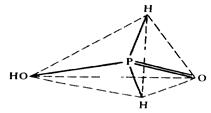
Structure (see the scheme):
The bond P—H existence in the molecule signifies that phosphorous acid is a dibasic one and its neutral salts are absent.
Н3РО3 is an acid of intermediate strength:
Н3РО3 Û Н+ + Н2РО3- k1 = 8.10-3
Н2РО3- Û Н+ + НРО32- k2 = 2.6.10-7
• It is a low active reducing agent and oxidizing properties are practically absent (Eо РО32-/РО43- = +0.28 V).
Hg(NO3)2 + H3PO3 + H2O = Hg + H3PO4 + 2HNO3
H3PO3 + I2 + H2O = H3PO4 + 2HI
• Н3РО3 can be an oxidising agent only with strong reducing agents:
Н3РО3 + 6Н (Zn+HCl) = РН3 + 3Н2О
• It disproportionates when heating
4Н3РО3 = 3Н3РО4 + РН3
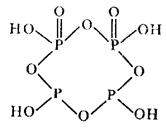
At heating dihydrogen phosphite salts of pyrophosphorous (diphosphorous) acid pyrophosphites can be obtained:
2NaH2PO3 = Na2H2P2O5 + H2O
Pyrophosphites hydrolyse when boiling:
Na2H2P2O5 + 3H2O = 2NaOH + 2H3PO3
Pyrophosphorous acid itself is of low stability. It structure is shown below:
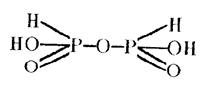
There is also metaphosphorous acid of polymeric structure (HPO2)n
Phosphoric acids. Р2О5 interaction with water depending on the number of associated molecules of H2O leads to the formation of a family of phosphoric acids.
|
|
|
Structure. Phosphoric acid exists in two polymeric forms:
· linear
· cyclic.
For instance, H3PO4 is a linear polymer, and (HPO3)n is a cyclical one. Let us consider some types of phosphoric acid. Some of them are formed at the beginning of a reaction (numbers indicate the order of appearance)
Tetrametaphosphoric 2. Tetrapolyphosphoric
Р4О10 + 2Н2О = (НРО3)4 (НРО3)4 + Н2О = Н6Р4О13
|
|
|
|
Дата добавления: 2021-03-18; просмотров: 41; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!