Nonstandard Amino Acids
The vast majority of proteins are assembled from the 20 amino acids listed above even though some of these may be chemically altered, e.g. by phosphorylation, at a later time.
However, two cases have been found where an amino acid that is not one of the standard 20 is inserted by a tRNA into the growing polypeptide.
- selenocysteine. This amino acid is encoded by UGA. UGA is still used as a chain terminator, but the translation machinery is able to discriminate when a UGA codon should be used for selenocysteine rather than STOP. This codon usage has been found in certain Archaea, eubacteria, and animals (humans synthesize 25 different proteins containing selenium).
- pyrrolysine. In several species of Archaea and bacteria, this amino acid is encoded by UAG. How the translation machinery knows when it encounters UAG whether to insert a tRNA with pyrrolysine or to stop translation is not yet known.
19) Structure of m RNA. 
5' cap[edit]
The 5' cap is a modified guanine nucleotide added to the "front" (5' end) of the pre-mRNA using a 5'-5'-triphosphate linkage. This modification is critical for recognition and proper attachment of mRNA to the ribosome, as well as protection from 5' exonucleases. It may also be important for other essential processes, such as splicing and transport.
Coding regions[edit]
Coding regions are composed of codons, which are decoded and translated (in eukaryotes usually into one and in prokaryotes usually into several) into proteins by the ribosome. Coding regions begin with the start codon and end with a stop codon. In general, the start codon is an AUG triplet and the stop codon is UAA, UAG, or UGA. The coding regions tend to be stabilised by internal base pairs, this impedes degradation.[6][7] In addition to being protein-coding, portions of coding regions may serve as regulatory sequences in thepre-mRNA as exonic splicing enhancers or exonic splicing silencers.
Untranslated regions[edit]
Untranslated regions (UTRs) are sections of the mRNA before the start codon and after the stop codon that are not translated, termed the five prime untranslated region (5' UTR) andthree prime untranslated region (3' UTR), respectively. These regions are transcribed with the coding region and thus are exonic as they are present in the mature mRNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and translational efficiency. The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs.
|
|
|
Poly(A) tail[edit]
The 3' poly(A) tail is a long sequence of adenine nucleotides (often several hundred) added to the 3' end of the pre-mRNA. This tail promotes export from the nucleus and translation, and protects the mRNA from degradation.
20) Describe the stage of aminoacids activation.
Amino acid activation refers to the attachment of an amino acid to its Transfer RNA (tRNA).
· Aminoacyl transferase binds Adenosine triphosphate (ATP) to amino acid.
· Aminoacyl transferase binds ATP-amino acid to tRNA. The ATP is used in this step.
Amino Acid Activation
During amino acid activation the amino acids (aa) are attached to their corresponding tRNA. The coupling reactions are catalysed by a group of enzymes called aminoacyl-tRNA synthetases (named after the reaction product aminoacyl-tRNA or aa-tRNA). The coupling reaction proceeds in two steps:
1. aa + ATP aa-AMP + PP, (pyrophosphate) 2. aa-AMP + tRNA aa-tRNA + AMP
The amino acid is coupled to the penultimate nucleotide at the 3’-end of the tRNA (the A in the sequence CCA) via an ester bond (roll over in illustration). The formation of the ester bond conserves a considerable part of the energy from the activation reaction. This stored energy provides the majority of the energy needed for peptide bond formation during translation.
Each of the 20 amino acids are recognized by its specific aminoacyl-tRNA synthetase. The synthetases are usually composed of one to four protein subunits. The enzymes vary considerably in structure although they all perform the same type of reaction by binding ATP, one specific amino acid and its corresponding tRNA.
The specificity of the amino acid activation is as critical for the translational accuracy as the correct matching of the codon with the anticodon. The reason is that the ribosome only sees the anticodon of the tRNA during translation. Thus, the ribosome will not be able to discriminate between tRNAs with the same anticodon but linked to different amino acids.
The error frequency of the amino acid activation reaction is approximately 1 in 10 000 despite the small structural differences between some of the amino acids.
|
|
|
21) List and characterize the main stages of translation. Imagine as a picture every stage of translation.
translation is the process in which cellular ribosomes create proteins. It is part of the process of gene expression. In translation, messenger RNA (mRNA) produced bytranscription is decoded by a ribosome complex to produce a specific amino acid chain, or polypeptide, that will later fold into an active protein. In bacteria, translation occurs in the cell's cytoplasm, where the large and small subunits of the ribosome are located, and bind to the mRNA. In eukaryotes, translation occurs across the membrane of the endoplasmic reticulum in a process called vectorial synthesis. Theribosome facilitates decoding by inducing the binding of tRNAs with complementary anticodon sequences to that of the mRNA. The tRNAs carry specific amino acids that are chained together into a polypeptide as the mRNA passes through and is "read" by the ribosome in a fashion reminiscent to that of a stock ticker and ticker tape.

22)Characterize the stages of glycolysis. Indicate the localization of this process in cell. What is the energy yield of glycolysis?
Glycolysis literally means "splitting sugars." In glycolysis, glucose (a six carbon sugar) is split into two molecules of a three-carbon sugar. Glycolysis yields two molecules of ATP (free energy containing molecule), two molecules of pyruvic acid and two "high energy" electron carrying molecules of NADH. Glycolysis can occur with or without oxygen. In the presence of oxygen, glycolysis is the first stage of cellular respiration. Without oxygen, glycolysis allows cells to make small amounts of ATP. This process is called fermentation.
Step 1
The enzyme hexokinase phosphorylates (adds a phosphate group to) glucose in the cell's cytoplasm. In the process, a phosphate group from ATP is transferred to glucose producing glucose 6-phosphate.
Glucose (C6H12O6) + hexokinase + ATP → ADP + Glucose 6-phosphate (C6H11O6P1)
Step 2
The enzyme phosphoglucoisomerase converts glucose 6-phosphate into its isomer fructose 6-phosphate. Isomers have the same molecular formula, but the atoms of each molecule are arranged differently.
Glucose 6-phosphate (C6H11O6P1) + Phosphoglucoisomerase → Fructose 6-phosphate (C6H11O6P1)
|
|
|
Step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to fructose 6-phosphate to form fructose 1, 6-bisphosphate.
Fructose 6-phosphate (C6H11O6P1) + phosphofructokinase + ATP → ADP + Fructose 1, 6-bisphosphate (C6H10O6P2)
Step 4
The enzyme aldolase splits fructose 1, 6-bisphosphate into two sugars that are isomers of each other. These two sugars are dihydroxyacetone phosphate and glyceraldehyde phosphate.
Fructose 1, 6-bisphosphate (C6H10O6P2) + aldolase → Dihydroxyacetone phosphate (C3H5O3P1) + Glyceraldehyde phosphate (C3H5O3P1)
Step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis.
Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1)
Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1)
Step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate. This occurs for both molecules of glyceraldehyde phosphate produced in step 5.
A. Triose phosphate dehydrogenase + 2 H- + 2 NAD+ → 2 NADH + 2 H+
B. Triose phosphate dehydrogenase + 2 P + 2 glyceraldehyde phosphate (C3H5O3P1) → 2 molecules of 1,3-bisphosphoglycerate (C3H4O4P2)
Step 7
The enzyme phosphoglycerokinase transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP. This happens for each molecule of 1,3-bisphosphoglycerate. The process yields two 3-phosphoglycerate molecules and two ATP molecules.
2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3-phosphoglycerate (C3H5O4P1) + 2 ATP
|
|
|
Step 8
The enzyme phosphoglyceromutase relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate.
2 molecules of 3-Phosphoglycerate (C3H5O4P1) + phosphoglyceromutase → 2 molecules of 2-Phosphoglycerate (C3H5O4P1)
Step 9
The enzyme enolase removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP). This happens for each molecule of 2-phosphoglycerate.
2 molecules of 2-Phosphoglycerate (C3H5O4P1) + enolase → 2 molecules of phosphoenolpyruvic acid (PEP) (C3H3O3P1)
Step 10
The enzyme pyruvate kinase transfers a P from PEP to ADP to form pyruvic acid and ATP. This happens for each molecule of PEP. This reaction yields 2 molecules of pyruvic acid and 2 ATP molecules.
2 molecules of PEP (C3H3O3P1) + pyruvate kinase + 2 ADP → 2 molecules of pyruvic acid (C3H4O3) + 2 ATP
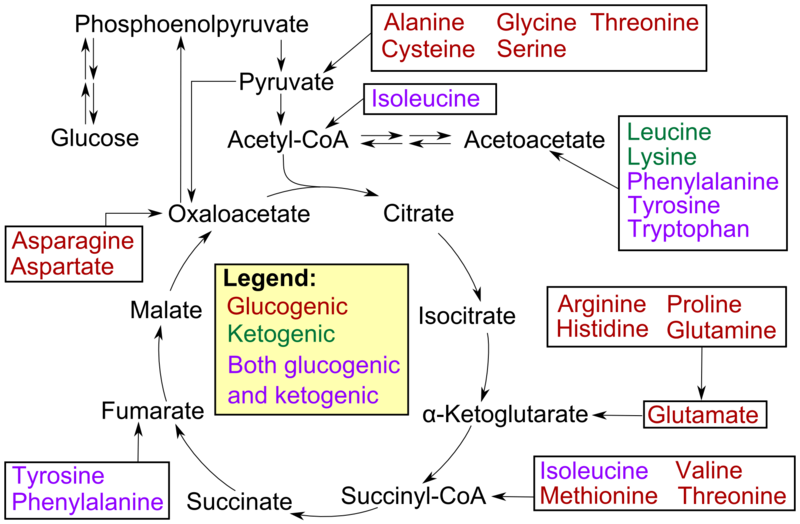
23) Open the biological significance of citric acid cycle. Bring the scheme according to which the Creb’s cycle is carried out.
The citric acid cycle — also known as the tricarboxylic acid cycle (TCA cycle), or the Krebs cycle —is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.
In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in thecytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.

24)gluconeogenesis
Gluconeogenesis (abbreviated GNG) is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as pyruvate, lactate, glycerol,glucogenic amino acids, and odd-chain fatty acids.
It is one of the two main mechanisms humans and many other animals use to keep blood glucose levels from dropping too low (hypoglycemia). The other means of maintaining blood glucose levels is through the degradation of glycogen (glycogenolysis).[1]
Gluconeogenesis is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms.[2] In vertebrates, gluconeogenesis takes place mainly in the liverand, to a lesser extent, in the cortex of kidneys. In ruminants, this tends to be a continuous process.[3] In many other animals, the process occurs during periods of fasting,starvation, low-carbohydrate diets, or intense exercise. The process is highly exergonic until ATP or GTP are utilized, effectively making the process endergonic. For example, the pathway leading from pyruvate to glucose-6-phosphate requires 4 molecules of ATP and 2 molecules of GTP. Gluconeogenesis is often associated with ketosis. Gluconeogenesis is also a target of therapy for type II diabetes, such as metformin, which inhibits glucose formation and stimulates glucose uptake by cells.[4] In ruminants, because metabolizable dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc.
In humans the main gluconeogenic precursors are lactate, glycerol (which is a part of the triacylglycerol molecule), alanineand glutamine. Altogether, they account for over 90% of the overall gluconeogenesis.[7] Other glucogenic amino acid as well as all citric acid cycle intermediates, the latter through conversion to oxaloacetate, can also function as substrates for gluconeogenesis.[8] In ruminants, propionate is the principal gluconeogenic substrate.
Дата добавления: 2016-01-05; просмотров: 10; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
