Plasmacoagulase activity S. а ureus in citrate plasma
Date__________________
Class 16
Topic: Infection and Infectious Process.
Questions to be discussed:
1. Definitions of infection, infection process, and disease.
2. Microorganisms, which can cause infection.
3. Microbial pathogenicity and virulence. Methods for virulence examination. Infection dose.
4. Microbial pathogenicity factors: adhesion, colonization, invasiveness, aggression, toxigenicity.
5. Basic classification of the bacterial toxins (endotoxins and exotoxins).
6. Characteristic features of the exotoxins. Classification of the exotoxins according to the mechanism of action.
7. Characteristic features of the endotoxins. Biological properties of the endotoxins. Genetic regulation of the pathogenicity factors.
8. Classifications of infections.
9. Types of clinical manifestation of the infection disease.
10. Typical stages (periods) of infection disease.
11. Epidemiological terminology (sporadic disease, endemic, epidemic).
12. Main elements of the infectious disease cycle or chain of infection (characteristic pathogen, source and/or reservoir of infection, mode and factors of transmission, susceptibility of the host, exit from the host).
13. Epidemiological classification of infections (anthroponoses, zoonoses, sapronoses).
Practical tasks:
1. Examine samples demonstrating the virulence factors produced by Staphylococcus aureus:
- hemolysin production on inoculated blood agar;
- lecitinase production inoculated on yolk-salt agar;
- plasmacoagulase production in citrate plasma;
Sketch out and label demonstrated properties of bacteria (Fig.1-3).
 |  |
Fig. 1. Haemolytic activity by S. аureus Fig. 2. Lecitinase activity S. аureus on blood agar on yolk-salt agar
Haemolytic activity by S. а ureus on blood agar
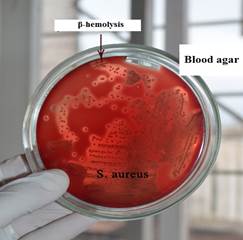
| Staphylococcus hemolysins are exotoxins (membrane-toxins) with enzymatic activity. Most importantly, the alpha-toxin, which causes destruction membrane of the erythrocyte. Its formation is determined by placing material from a patient with staphylococcal infection on blood agar (meat-peptone agar with 5% blood). Around the staphylococcus colonies, zones of complete enlightenment of agar (β-hemolysis) are formed. |
Lecitinase activity S. а ureus on yolk-salt agar
|
|
|
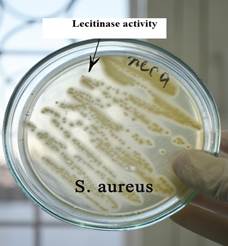
| The determination of lecithinase is carried out on yolk-salt agar containing meat-peptone agar with 10% sodium chloride and chicken yolk, which is the source of lecithin. On the yolk-salt agar around the grown colonies of staphylococcus (yellow), a zone of turbidity is formed due to the hydrolysis of phospholipid - lecithin. Lecithin is a mixture of phospholipids - components of the cell membranes needed by every cell in the body. It is also contained in the myelin sheath of nerve cells, the membrane of muscle cells, is a hepatoprotector and a component of the endocrine glands. Therefore, the enzyme lecithinase is an important factor in the pathogenicity of Staphylococcus aureus. |
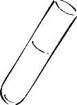

Fig. 3. Plasmacoagulase activity S. аureus in citrate plasma
Plasmacoagulase activity S. а ureus in citrate plasma

| Staphylococcus aureus produces an enzyme of pathogenicity - plasmacoagulase. Staphylococcus penetrates from the surface of the skin into the vessels, where, under the action of plasmacoagulase the formation of a fibrin clot from fibrinogen. This clot protecte the bacteria from factors of non-specific resistance - phagocytosis and complement system. To study this property, the isolated staphylococcus culture is introduced into a tube with rabbit citrate plasma. The tubes are placed in a thermostat at 37°C and the time of plasma clotting is noted. The deadline for recording the result is 24 hours. |
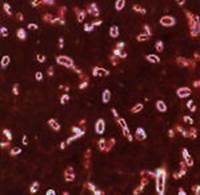
2. Find capsule in a Hince-Burri’s stained smear of Klebsiella pneumonia pure culture (microscopic examination of the slide) and draw capsules.
 |
| 
|
|
|
|
Fig.4. Capsules of Klebsiella pneumoniae Hince-Burri’s staining
3. Examine a plate demonstrating evaluation of Corynebacteium diphtheriae toxigenicity by immunodiffusion (Elek’s test) in special solid culture media. Draw a picture.
 | |||
 | |||
Fig. 5. Evaluation of toxigenicity of Corynebacteium diphtheriae by imunodiffusion method.
Label toxigenic bacterial cultures.
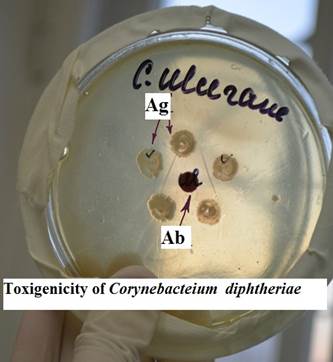
| The reaction is based on the interaction of antibodies and antigens in an agar gel as a result of counter diffusion and the formation of visible precipitation lines. In this reaction, the antigen is exotoxin of Corynebacterium diphtheria, the antibody is the immune antitoxic serum of animals. The reaction is used to determine the release of toxin (toxigenic properties) of diphtheria pathogen isolated from patients or carriers. Translucent precipitation lines are visible on a Petri dish between bacterial colonies and a disk with antitoxic serum. The reaction is controlled by laboratory toxigenic and non-toxigenic strains of corynebacteria. |
Дата добавления: 2021-07-19; просмотров: 44; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
