Microleakage and shear bond strength
Table 1 shows the mean (±SD) of the proportion of microleakage values in each group. There were no significant differences between the groups (P = 0.939). However, the differences between groups in mean (±SD) SBS were significant (Table 2). Mean SBS in group 2 was significantly greater than in groups 1, 3 and 4 (all P < 0.05). There were no significant differences in SBS between group 5 and the other groups. The results of failure mode tests showed that mixed failure was the most frequent mode in all groups.
| Group | Median (mean ± SD) | Mean rank | p -value |
| 1. Etch + FS | 0.0 (0.09 ± 0.17) | 52.2 | 0.939 |
| 2. Etch + 0.15% nano-HA + FS | 0.0 (0.09 ± 0.18) | 52.3 | |
| 3. Etch + 0.03% nano-HA + FS | 0.0 (0.08 ± 0.23) | 49.7 | |
| 4. Etch + nano-HA 0.15% + SHMP 0.05% + FS | 0.0 (0.08 ± 0.23) | 50.9 | |
| 5. Etch + nano-HA 0.03% + SHMP 0.01% + FS | 0.0 (0.05 ± 0.13) | 47.3 |
| Group | Mean ± SD | Duncan’s testa |
| 1. Etch + FS | 14.6 ± 2.5 | A |
| 2. Etch + 0.15% nano-HA + FS | 16.8 ± 2.7 | B |
| 3. Etch + 0.03% nano-HA + FS | 14.2 ± 2.1 | A |
| 4. Etch + nano-HA 0.15% + SHMP 0.05% + FS | 14.0 ± 2.2 | A |
| 5. Etch + nano-HA 0.03% + SHMP 0.01% + FS | 15.1 ± 2.5 | AB |
| p-value | 0.010 |
Sealant penetration
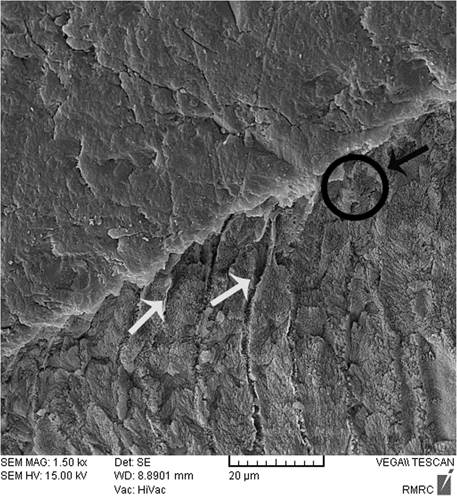
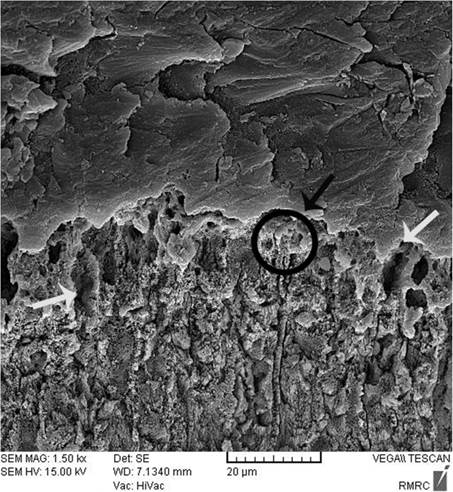
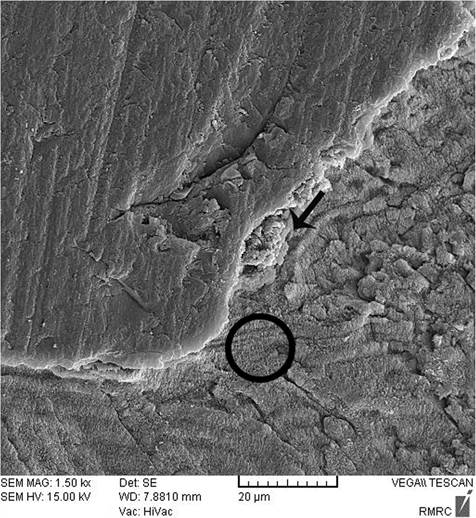
Sealant penetration was investigated with SEM in the deepest part of the pit and fissures in group 1. The prism-like appearance well was defined due to the demineralization process and effect of etching. The spaces were filled with resin sealant (Fig. 2). Applying nano-HA 0.15% created a layer that covered the surface and penetrated into parts of the etched enamel (Fig. 3). The use of nano-HA 0.03% led to resin infiltration in parts of the fissures, although some parts showed no penetration (Fig. 4). Applying SHMP in group 4 (Fig. 5) and group 5 (Fig. 6) led to increased dispersal of the nanoparticles over a larger area, with no increase in the depth of infiltration in the fissures.
|
|
|

Mineral content
| Condition | |||||
| Group | Baseline | DEM | REM | Total | p -value |
| 2 | 2.4 ± 0.1 | 1.8 ± 0.2 | 2.1 ± 0.3 | 2.1 ± 0.1 | 0.582 |
| 3 | 2.4 ± 0.1 | 1.8 ± 0.1 | 2.3 ± 0.3 | 2.1 ± 0.1 | |
| 4 | 2.4 ± 0.0 | 1.8 ± 0.3 | 2.1 ± 0.4 | 2.1 ± 0.2 | |
| 5 | 2.4 ± 0.1 | 1.8 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.1 | |
| Total | 2.4 ± 0.1 | 1.8 ± 0.2 | 2.2 ± 0.3 | p-value< 0.001 | |
Mineral contents in each sample at all three steps were measured by EDS. There was no significant interaction between compositions and conditions (P = 0.794). In other words, the effect of different conditions on the Calcium (Ca)/Phosphate (P) ratio did not depend on the type of composition used (Fig. 7). There were significant differences in Ca/P weight percentage (Wt%) ratio between baseline, demineralized and remineralized enamel (P < 0.001) (Table 3). The highest ratio was recorded for sound enamel and the lowest for demineralized enamel. There were no significant differences in Ca/P Wt% ratio between groups 2, 3, 4 and 5 regardless of the conditions (P = 0.582).
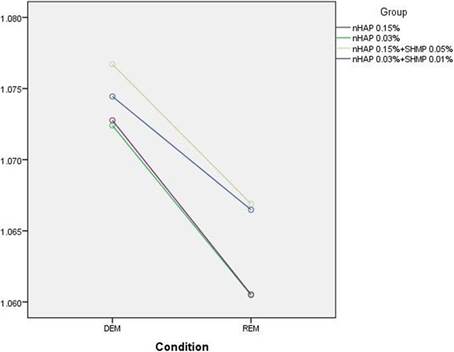
The standardless EDS value with ZAF correction for matrix effects (EDS-ZAF) was also recorded. There was no significant interaction between compositions and conditions (P = 0.385). In other words, the effect of changing ZAF for Ca/P from demineralization to remineralization did not depend on the type of composition used (Fig. 8).  Рис.8
Рис.8
The results showed significant differences in ZAF Ca/P ratios between all groups (P < 0.001) and between groups 2 to 5 (P = 0.004). There were significant differences in ZAF-corrected Ca/P ratios between groups 4 and 3 (P = 0.014) and groups 4 and 5 (P = 0.019). However, there was no significant difference between groups 4 and 2 (P = 0.857). Also regardless of the group, the ratio in remineralized enamel was significantly higher compared to after demineralization (P < 0.001). Table 4 shows the mean ZAF Ca/P ratios in different conditions and groups
|
|
|
|
| ||||
| Group | DEM | REM | Total | p -value |
| 2 | 1.074 ± 0.006 | 1.072 ± 0.007 | 1.070 ± 0.005 AB | 0.004 |
| 3 | 1.077 ± 0.006 | 1.073 ± 0.007 | 1.066 ± 0.004 A | |
| 4 | 1.074 ± 0.006 | 1.066 ± 0.005 | 1.072 ± 0.004 B | |
| 5 | 1.060 ± 0.003 | 1.067 ± 0.006 | 1.067 ± 0.003 A | |
| Total | 1.074 ± 0.006 | 1.064 ± 0.005 | p-value< 0.001 | |
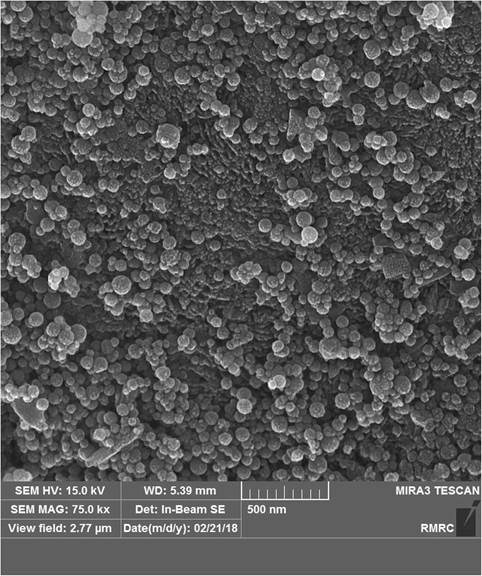
Nanoparticle micromorphology
Nano-HA particles were spherical in all the interventional groups. The structure was porous, and large numbers of nanoparticles were dispersed on the surface in group 2 (Fig. 9). The FESEM images for group 3 revealed a similar appearance except with fewer nanoparticles and less particle clustering (Fig. 10). Applying SHMP (groups 4 and 5) resulted in nano-HA dispersal over a larger surface area and less clustering (Figs. 11 and 12) compared to the groups without SHMP.



Discussion
The present results showed no significant difference between intervention groups and the control group (FS alone), which was in accordance with our null hypothesis. The higher concentration of nano-HA (0.15%) led to greater SBS compared to the control group; however, there was no significant difference between the control group and the group with the lower concentration (0.03%) of nano-HA. Thus the null hypothesis (H0) was partially rejected.
|
|
|
Guidelines have recommended using a pit and fissure sealant on sound intact enamel and noncavitated (incipient caries) lesions [2, 3]. Some materials, such as nanoparticles, have been found to increase enamel remineralization in sealant therapy [13,14,15,16].
Nano-HA [Ca10(PO4)6(OH)2] is similar in structure to the main mineral component of teeth and bone, and products containing nanoparticles have led to enhanced precipitation of calcium and phosphate ions in the tooth structure [14, 20, 28]. Some studies reported that a suspension containing 10% nano-HA particles (10–20 nm diameter) enhanced remineralization of the superficial layer in initial caries lesions to a depth of 20–40 μm. However, little remineralization was seen in the body of the lesion [29, 30]. One earlier study found that nano-HA (20 nm size and dimension up to 100–150 mm) enhanced remineralization in the subsurface of initial lesions, particularly in dentin compared to enamel [23]. In the present study the mean diameter of nano-HA was 10.67 nm.
Factors that influence the success of sealant treatment include marginal adaptation between the tooth and fissure sealant, and bond strength of the materials [9, 27]. In the present study all groups showed some microleakage, which is consistent with previous work that found no sealant materials were able to completely eliminate microleakage between the tooth and sealant [30]. Several factors influence microleakage along the sealant and enamel surface, e.g. pit and fissure morphology, sealant viscosity and method of FS application, all of which impact sealant infiltration [31]. Other factors include the enamel pretreatment technique [6, 7], intensity of light curing [31], and mechanical properties of the sealant materials [31, 32]. Resin polymerization shrinkage as well as thermal dimensional changes in fissure sealants [33] may also lead to microleakage and gap formation. We observed no significant differences between the interventional groups (groups 2 to 5) and the control group without nano-HA application. This result is consistent with a previous study in which the use of products containing acetone prior to sealant application led to similar results for sealant adaption compared to conventional acid etching [30]. These findings may be due to the presences of acetone as a solvent in the nano-HA solution. Acetone is a colorless liquid that evaporates rapidly and can be mixed with most organic solvents and with water. Nano- HA solutions containing acetone and water, therefore, may improve resin infiltration into the demineralized enamel [24]. Another explanation for these results may be the lack of a significant effect of nanoparticles on the sealing capacity of the sealant.
|
|
|
In the present study SBS differed significantly between group 2 (nano-HA) and groups 1 (control), 3 and 4. This may reflect the ability of the hydrophobic resin sealant to enhance surface wettability of the etched enamel as a result of nano-HA properties [16] and the presence of acetone in the solution. Hydroxyapatite “acts as a drug delivery carrier due to superior adsorptive properties”, and this may in turn increase bond strength [14]. Another explanation for the increased bond strength may be the creation of a reactive layer between the resin sealant and nano-HA. In group 2 the higher concentration of nanoparticles (0.15%) may have contributed to the greater SBS. In agreement with our study, Borges et al. showed that applying casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) products beneath the fissure sealant promoted bond strength between the resin sealant and the enamel [12].
After enamel demineralization, the loss of minerals such as calcium and phosphorus results in voids or gaps on the tooth surface. The acid etch technique also creates microporosities on the enamel surface. Both processes provide spaces that facilitate resin infiltration and suitable bonding between the resin and tooth. When nano-HA solution was used, the ionic form of Ca and P enters the porous enamel and provides seeds for remineralization [25, 28, 34, 35]. SEM images in the present study showed differences between groups in the degree of penetrations of the resin sealant into these spaces.
Energy dispersive X-ray spectroscopy is a chemical elemental microanalysis technique to measure mineral content at the ultrastructural level, and has been used to investigate the remineralization capacity of different materials [14, 17, 24]. In the present study we used FESEM instead of SEM to obtain more detailed structural data of higher quality and resolution [35]. Our analysis of EDS data focused on changes in Wt% of Ca, P and the Ca/P ratio, given that these ions are important indicators of the effect of nano-HA on enamel remineralization. To enhance the accuracy of our EDS analysis, we compared three portions of each tooth sample at each of three steps: baseline (sound enamel, as a reference), after demineralization, and after remineralization. In addition we obtained the ZAF-corrected Ca/P ratio to further increase the accuracy of the EDS results.
Our EDS results showed that nano-HA released Ca and P ions onto the demineralized surface, in accordance with previous studies [18, 24]. The lowest Ca/P Wt% ratios were seen (not unexpectedly) in demineralized enamel. There were no significant differences in this ratio between groups – a finding consistent with earlier EDS results for Ca/P ratio in a study of nano-HA in demineralized dentin [24]. This may explain, in part, our finding that Wt% Ca/P did not differ among our experimental groups. Although SHMP and acetone increase solubility of nano-HA and would thus be expected to enhance nanoparticle infiltration [24], SHMP did not increase nanoparticle infiltration in deeper parts of the enamel in the present study.
In the present study the ZAF-corrected Ca/P ratio in demineralized enamel was higher than in the interventional groups, as a foreseeable result of the demineralization process. Among the interventional groups, the ZAF-corrected ratio in group 4 was significantly higher than in groups 3 and 5. This may be related to the higher values of P (i.e. the higher concentration of SHMP) in group 4 (0.15% nano-HA + 0.05% SHMP). In dentistry, SHMP is used as a deflocculant to limit nanoparticle agglomeration or reduce the size of nano-HA agglomerates to less than 5 nm [24]. Our SEM observations showed that in groups 4 and 5 (both with SHMP) the nanoparticles were better dispersed on the surface of the sealant–enamel interface than in groups 2 and 3 (without SHMP). In group 1 (control without nano-HA) the sealant penetrated to the deepest parts of pits and fissures. When the higher concentration of nanoparticle was used (group 2), a layer of nano-HA was seen on the treated enamel – a finding consistent with an earlier study with tricalcium phosphate nanoparticles [15]. Group 4 also used nano-HA at 0.15%, together with SHMP, and this combination may have influenced our SBS and SEM results. However, the lack of earlier studies centered on nanoparticles in treated enamel precludes further comparisons with previous findings.
Nanoparticle micromorphology was investigated in FESEM images, which showed that they were spherical as reported in a previous study [18]. In group 2 (with the higher concentration of nano-HA), FESEM also showed larger amounts of nanoparticles dispersed on the surface area. In groups 4 and 5 (with SHMP), FESEM images showed more nanoparticles which were well dispersed on a larger surface area compared to groups 2 and 3. Earlier studies found that nonagglomerated particles are better able to penetrate into the surface and prevent nanoparticle precipitation [17, 25]. However, nanoparticle penetration into the deeper parts of the tooth was insufficient, as also reported by others [15].
A potential limitation of this study was that laboratory tests may not accurately reflect clinical conditions. To reduce the influence of confounding factors, we used thermocycling, which replicates actual clinical procedures. Another limitation is the lack of studies designed to investigate the effects of nanoparticles used prior to sealant application; this precluded comparisons between our findings and those of other studies. A further potential limitation is the approach we used to prepare the materials tested in each group. Therefore a pilot analysis was done to determine the appropriate concentrations of nano-HA and SHMP for testing before the experimental specimens were used for data collection and analysis. Our pilot study was based essentially on the methods of Besinis et al., and we implemented their 3:1 ratio of nano-HA to SHMP [24, 25]. However, we tested different concentration of nano-HA to obtain more complete results. The results of the present study support our hypothesis that nano-HA would be able to remineralize enamel and would enhance SBS without influencing the sealant’s sealing ability. Finally, we recommend additional in vitro and in vivo studies to determine the advantages and drawbacks of applying nano-HA before the fissure sealant in terms of clinical outcomes.
Conclusions
The use of nano-HA before fissure sealant application may be an effective method to fill porous spaces in demineralized enamel pits and fissures, and to enhance remineralization compared to demineralized enamel. Moreover, the higher concentration of nano-HA (0.15%) led to increased shear bond strength of the sealant compared to conventional acid etching and sealant application. Both concentrations of nano-HA (0.15 and 0.03%) with or without SMHP led to enhanced remineralization compared to demineralized enamel.
Abbreviations
Ca:
Calcium
CPP-ACP:
Casein phosphopeptide-amorphous calcium phosphate
EDS:
Energy-dispersive X-ray spectroscopy
FESEM:
Field emission scanning electron microscope
FS:
Pit and fissure sealant
Nano-HA:
Nanohydroxyapatite
P:
Phosphate
SBS:
Shear bond strength
SCi:
Silicon carbide paper
SEM:
Scanning electron microscopy
SHMP:
Sodium hexametaphosphate
References
Дата добавления: 2020-11-29; просмотров: 105; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
