Table 1.1 Physical properties of some liquefied gases
| Liquefied gas | Vapour pressure at 37.8°C (bars absolute) | Boiling point at atmospheric pressure (°C) |
| Methane | Gas* | -161.5 |
| Propane | 12.9 | -42.3 |
| n-Butane | 3.6 | -0.5 |
| Ammonia | 14.7 | -33.4 |
| Vinyl chloride | 5.7 | -13.8 |
| Butadiene | 4.0 | -5 |
| Ethylene oxide | 2.7 | +10.7 |
*The critical temperature of methane is -82.5°C while the critical pressure is 44.7 bars. Therefore, at a temperature of 37.8°C it can only exist as a gas and not as a liquid.
On the basis of the above IMO definition, ethylene oxide (see Table 1.1) would not qualify as a liquefied gas. However, it is included in the International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (the IGC Code) because its boiling point at atmospheric pressure is so low that it would be difficult to carry the cargo by any method other than those prescribed for liquefied gases.
Likewise, chemicals such as diethyl ether, propylene oxide and isoprene are not strictly liquefied gases but they have high vapour pressures coupled with health and flammability hazards. As a result of such dangers these chemicals, and several similar compounds, have been listed jointly in both the IGC Code and the Bulk Chemical Codes. Indeed, when transported on chemical tankers, under the terms of the Bulk Chemical Codes, such products are often required to be stowed in independent tanks rather than in tanks built into the ship's structure.
The listing of liquefied and chemical gases given in the IGC Code is shown in Appendix 2.
1.2 LIQUEFIED GAS PRODUCTION
To assist in understanding the various terms used in the gas trade, this section discusses the manufacture of liquefied gases and describes the main gas carrier cargoes transported by sea. It is first of all necessary to differentiate between some of the raw materials and their constituents and in this regard the relationships between natural gas, natural gas liquids (NGLs) and Liquefied Petroleum Gases (LPGs) is shown in Figure 1.1.
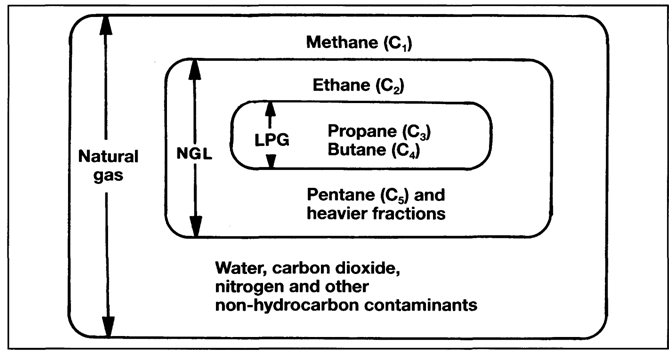
Figure 1.1 Constituents of natural gas
1.2.1 LNG production
Natural gas may be found in:
· Underground wells, which are mainly gas bearing (non-associated gas)
· Condensate reservoirs (pentanes and heavier hydrocarbons)
|
|
|
· Large oil fields (associated gas)
In the case of oil wells, natural gas may be either in solution with the crude oil or as a gas-cap above it.
Natural gas contains smaller quantities of heavier hydrocarbons (collectively known as natural gas liquids — NGLs). This is in addition to varying amounts of water, carbon dioxide, nitrogen and other non-hydrocarbon substances. These relationships are shown in Figure 1.1.
The proportion of NGL contained in raw natural gas varies from one location to another. However, NGL percentages are generally smaller in gas wells when compared with those found in condensate reservoirs or that associated with crude oil. Regardless of origin, natural gas requires treatment to remove heavier hydrocarbons and non-hydrocarbon constituents. This ensures that the product is in an acceptable condition for liquefaction or for use as a gaseous fuel.
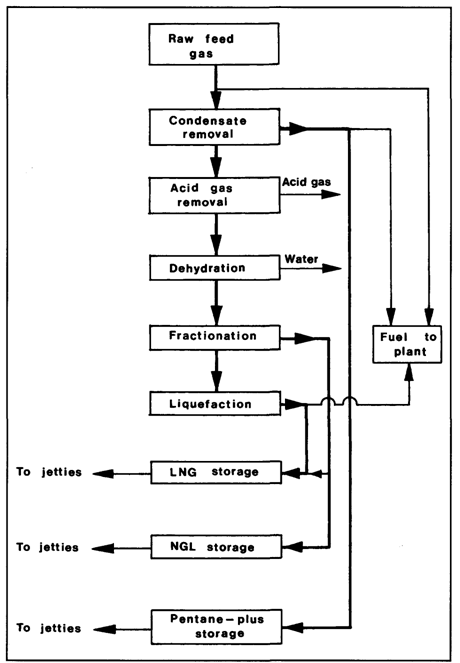
Figure 1.2 is a typical flow diagram for a liquefaction plant used to produce liquefied natural gas (LNG). The raw feed gas is first stripped of condensates. This is followed by the removal of acid gases (carbon dioxide and hydrogen sulphide). Carbon dioxide must be removed as it freezes at a temperature above the atmospheric boiling point of LNG and the toxic compound hydrogen sulphide is removed as it causes atmospheric pollution when being burnt in a fuel. Acid gas removal saturates the gas stream with water vapour and this is then removed by the dehydration unit.
Дата добавления: 2018-02-28; просмотров: 506; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
