Characterize crystalline, amorphocrystalline and liquid crystal polymers. Give examples
Supramolecular structure of polymers. Characterize ordered and disordered polymers. Give examples. 2 Characterize crystalline, amorphocrystalline and liquid crystal polymers. Give examples. 3 Structural architecture of branched polymers. Characterize randomly branched polymer chains (or trees). 4 Characterize chain architecture of linear and cross-linked polymers. Give examples. 5 Characterize architecture of cyclic polymers. Apply graph theory to analyze topology of complex cycles. 6 Structural architecture of branched polymers. Characterize rot-rot and H-polymers. 7 Structural architecture of branched polymers. Characterize comb polymers. 8 Structural architecture of branched polymers. Characterize hyperbranched polymers. 9 Structural architecture of branched polymers. Characterize dendrimers. 10 Structural architecture of branched polymers. Characterize star polymers. 11 Evaluate the differences between molecular structure of low and high molecular compounds. Give examples. 12 Evaluate the differences between topological structure of low and high molecular compounds. Give examples. 13 Colloid-dispersed structural organization of polymers. Evaluate the differences between colloidal structure of low and high molecular compounds. 14 Discuss methods for the synthesis of well-defined block copolymers via reversible addition fragmentation chain transfer polymerization in the presence of mono- and difunctional RAFT-agents. 15 Discuss methods for the synthesis of well-definedblock copolymers via atom transfer radical polymerization. 16 Discuss methods for the synthesis of well-defined block copolymers via nitroxide mediated radical polymerization. Compare two basic strategies of NMP initiation. 17 Discuss methods for the obtaining of graft copolymers by grafting-onto, grafting-from and grafting-through approaches. 18 Discuss methods for the synthesis of well-defined multi-block copolymers by alternation copolymerization. 19 Discuss methods for the synthesis of star polymers by “core first” and “arm first” approaches. 20 Discuss methods for the synthesis of block copolymers by living anionic polymerization. Evaluate advantages and disadvantages of these methods in comparison with main techniques of living radical copolymerization. 21 Propose one-stage methods for the synthesis of styrene-maleic anhydride diblock copolymers using the RAFT technique. Draw appropriate schemes and formulas. 22 Propose two-stage methods for the synthesis of triblock copolymers using the RAFT technique. Draw appropriate schemes and formulas. 23 Synthesize temperature- and redox-responsive multi-block copolymers of poly(N-isopropylacrlamide) (PNIPAAM) and poly(dimethylaminoethylmethacrylate) (PDMAEMA) by RAFT polymerization. Draw appropriate schemes and formulas. 24 Propose method for the synthesis brush type graft copolymers via ATRP approach. 25 Propose method for the synthesis of polystyrene grafted chitosan via NMP using chitosan-TEMPO macroinitiator. 26 Propose method for the synthesis of styrene-acrylic acid amphiphilic diblock-gradient copolymer via nitroxide-mediated polymerization. 27 Propose method for the synthesis of styrene-acrylic acid amphiphilic triblock-gradient copolymer via nitroxide-mediated polymerization. 28 Synthesize 4-arm poly(2-vinylpyridine) star polymer using bromomethyl benzene derivative as linking agent. 29 Propose one-stage methods for the synthesis of styrene-maleic anhydride triblock copolymers using the RAFT technique. Draw appropriate schemes and formulas. 30 Synthesize 3-arm star-block copolymer based on (polystyrene-b-polyisoprene) diblock copolymer using suitable linking agent.
|
|
|
Supramolecular structure of polymers. Characterize ordered and disordered polymers. Give examples.
Supramolecular polymers are a kind of polymers whose monomeric units hold together via highly directional and reversible non-covalent interactions. Unlike conventional bonded polymers, supramolecular polymers engage in a variety of non-covalent interactions that define their properties. These interactions include hydrogen bonding, π-π interaction, metal coordination, and host-guest interaction. Owing to the presence of these reversible noncovalent interactions, supramolecular polymers exhibit dynamic properties such as self-healing.
The mechanism of noncovalent polymerization in supramolecular chemistry is highly dependent on the interactions that play their part in the self-assembly process.
In contrast to covalent bonds, noncovalent interactions depend on temperature and concentration, thereby affecting the degree of polymerization. The mechanisms of supramolecular polymerizations can be divided in three major classes, these being isodesmic, cooperative, or ring-chain equilibria. Isodesmic polymerizations occur when the strength of noncovalent interactions between monomers is unaffected by the length of the chain. Because each addition is equivalent, no critical temperature or concentration of monomers is required for the polymerization to occur. Instead, the length of the polymer chains rises as the concentration of monomers in the solution is increased, or as the temperature decreases. The ring-chain mechanism is characterized by an equilibrium between closed rings and linear polymer chains. In this mechanism, below a certain monomer concentration the ends of any small polymer chain react with each other to generate closed rings. Above this critical concentration, linear chain formation becomes more favored, and polymer growth is initiated.
|
|
|
The degree of polymerization changes abruptly once the critical conditions are reached. The critical polymerization concentration is largely dependent on the length and rigidity of the monomers. Especially at low concentrations, the presence of cyclic oligomers can drastically influence the macroscopic properties.
The degree of polymerization changes abruptly once the critical conditions are reached.

The critical polymerization concentration is largely dependent on the length and rigidity of the monomers. Especially at low concentrations, the presence of cyclic oligomers can drastically influence the macroscopic properties. Cooperative polymerizations occur in the growth of ordered supramolecular polymers in which there are additional interactions present besides the formation of linear polymers, such as those that form helices. This involves two distinct phases of self-assembly: a less favored nucleation phase followed by a favored polymerization phase. In this mechanism, the noncovalent bonds between monomers are weak, hindering the initial polymerization. After the formation of a nucleus of a certain size, the association constant is increased, and further monomer addition becomes more favored, at which point the polymer growth is initiated. Long polymer chains will form only above a minimum concentration of monomer and below a certain temperature, resulting in a sharp transition from a regime dominated by free monomers and small aggregates to a regime where almost all of the material is present as large polymers.
|
|
|
Characterize crystalline, amorphocrystalline and liquid crystal polymers. Give examples.
Crystalline polymers – polymers in the crystalline state. They have greater strength, less fluidity, the ability to form highly oriented structures than amorphous polymers. Crystallization leads to a change in the optical, thermal, dielectric, and other properties of polymers. Crystalline polymers, as well as low molecular weight crystalline substances, can exist in various crystal-chemical modifications. Crystalline polymers have high fluidity, but are very sensitive to the slightest changes in the thermal regime.
Crystalline polymers: polyethylene, polyamides, polypropylene, polyformaldehyde.
Amorphous-crystalline polymers are in their behavior between two extreme cases. The more the ability of the samples to crystallize and the higher the degree of crystallinity, the closer the curve resembles the curve for polymer crystals and vice versa, the lower the degree of crystallinity, the more the curve resembles the behavior of amorphous polymers. Crystalline and amorphous-crystalline polymers can only be thermoplastic.
Amorphous-crystalline polymer: polyvinyl chloride(PVC).
Liquid crystal polymer is a class of highly crystalline thermoplastics. They contain benzene rings in polymer chains, which are rodlike structures organized by large parallel matrices. Distinctive features: This material is characterized by lightness and strength.
3.Structural architectureof branchedpolymers.Characterize randomlybranched polymerchains (or trees).
According to the topology, the geometry of the skeleton of the polymer macromolecule is divided into the following types: linear, crosslinked and branched. Branched polymers consist of macromolecules, the main chain of which, unlike linear, contains arbitrarily located lateral branches of several atoms to the size of the main chain. The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center. Branched polymers containing branched branches in each link, for example polyhexadecyl acrylate, also include branched polymers:
|
|
|

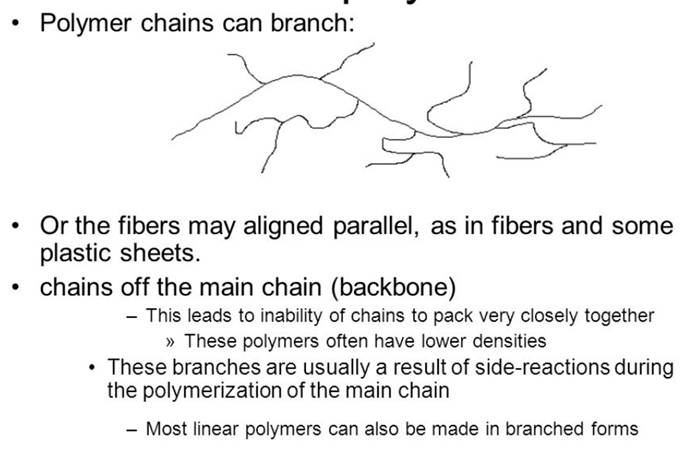

4)Characterize chainarchitectureof linearandcross-linkedpolymers. Give examples.
Polymer architecture in polymer science relates to the way branching leads to a deviation from a strictly linear polymer chain. Branching may occur randomly or reactions may be designed so that specific architectures are targeted. It is an important microstructural feature. A polymer's architecture affects many of its physical properties including solution viscosity, melt viscosity, solubility in various solvents, glass transition temperature and the size of individual polymer coils in solution.

An effect related to branching is chemical crosslinking - the formation of covalent bonds between chains. Crosslinking tends to increase Tg and increase strength and toughness. Among other applications, this process is used to strengthen rubbers in a process known as vulcanization, which is based on crosslinking by sulfur. Car tires, for example, are highly crosslinked in order to reduce the leaking of air out of the tire and to toughen their durability. Eraser rubber, on the other hand, is not crosslinked to allow flaking of the rubber and prevent damage to the paper. Polymerization of pure sulfur at higher temperatures also explains why sulfur becomes more viscous with elevated temperatures in its molten state. A polymer molecule with a high degree of crosslinking is referred to as a polymer network. A sufficiently high crosslink to chain ratio may lead to the formation of a so-called infinite network or gel, in which each chain is connected to at least one other.
With the continual development of Living polymerization, the synthesis of polymers with specific architectures becomes more and more facile. Architectures such as star polymers, comb polymers,brushpolymers, dendronized polymers, dendrimers and Ring polymers are possible. Complex architecture polymers can be synthesized either with the use of specially tailored starting compounds or by first synthesising linear chains which undergo further reactions to become connected together. Knotted polymers consist of multiple intramolecular cyclization units within a single polymer chain.
In general, the higher degree of branching, the more compact a polymer chain is. Branching also affects chain entanglement, the ability of chains to slide past one another, in turn affecting the bulk physical properties. Long chain branches may increase polymer strength, toughness, and the glass transition temperature (Tg) due to an increase in the number of entanglements per chain. A random and short chain length between branches, on the other hand, may reduce polymer strength due to disruption of the chains' ability to interact with each other or crystallize.
An example of the effect of branching on physical properties can be found in polyethylene. High-density polyethylene (HDPE) has a very low degree of branching, is relatively stiff, and is used in applications such as bullet-proof vests. Low-density polyethylene (LDPE), on the other hand, has significant numbers of both long and short branches, is relatively flexible, and is used in applications such as plastic films.
Dendrimers are a special case of branched polymer where every monomer unit is also a branch point. This tends to reduce intermolecular chain entanglement and crystallization. A related architecture, the dendritic polymer, are not perfectly branched but share similar properties to dendrimers due to their high degree of branching.
The degree of branching that occurs during polymerisation can be influenced by the functionality of the monomers that are used For example, in a free radical polymerisation of styrene, addition of divinylbenzene, which has a functionality of 2, will result in the formation of branched polymer.
5. Characterize architectureof cyclicpolymers. Apply graph theory to analyze topology of complex cycles.
The first attempts to synthesize cyclic polymers involved a ring-open chain equilibrium based on backbiting reactions of poly(dimethylsiloxanes). However, this method was limited to low molecular weight and polydisperse cyclic polymers and was characterized by the inability to isolate the corresponding linear precursor in order to prove the cyclic structure by comparing the properties. Now a days living polymerization processes leading to narrow molecular weight distribution polymers are generally preferred. The linear precursor of the cyclic polymer has either two identical or two different functional groups capable of reacting with each other. In the first case, an R,ω-homodifunctional macromolecule was synthesized first, followed by the reaction with an appropriate difunctional linking agent. In the second case, an R,ω-heterodifunctional macromol- ecule was prepared by using functional protected initiator and by neutralizing the living anion with the appropriate linking agent containing another protected group. The cyclic structure is formed by the coupling reaction of the two reactive groups.
 |
The first case is shown below schematically:
Besides the intramolecular reaction, several inter- molecular reactions can occur: 
These reactions lead to undesirable, high molecular weight polycondensates, which are either linear or cyclic and should be removed from the low molecular weight desirable cyclic product.

In the second case, the cyclization requires an activation step: 
6.Structural architectureof branchedpolymers.Characterize rot-rot and H-polymers.
The key feature of polymers is that they are very large molecules made up of long sequences of relatively simple chemical units. Most polymeric materials can be thought as consisting of individual, although very large molecules. In some cases the polymer chains are made up of linear sequences of monomeric units:

but other types of chain architecture are possible and have been exploited commercially to tailor the properties of polymers.
For example, in branched polymers

long chains are joined to the backbone at various points along the main chain. The existence of branches and their lengths have a profound effect on the physical properties. Polyethylene (PE) can be commercialised in three main different forms: low density PE (LDPE), linear low density polyethylene (LLDPE) and high density polyethylene (HDPE). All these materials have the same chemical structure but differ in degree of branching.
The presence of even short branches affects the ability of the polymer to crystallize. As a consequence, as branching increases the melting temperature decreases and so does the density of the material, indicating that packing becomes less efficient.
Some of the properties of LDPE, LLDPE and HDPE are summarised in the table below.
Branching also affects the mechanical properties of a material such as its tensile strength (which is defined as the ability of a material to withstand a tensile force).


In addition, to linear and branched polymers, other types of chain architecture lead to materials with interesting physical properties. For example, star polymers

have found use as viscosity modifiers in motor oil.
The rheological behavior of branched polymers contrasted with that linear melta motivates discussion of a molecular model of a melt of H-polymers. Concentrating on the dynamics of the ‘backbone’ we derive and discuss the linear viscoelastic properties in the light of experiments by Roovers, the model extended to treat deformation, with path length extension treated in self-consistent way, and compared with rheological behavior of branched LDPE.
7 Structural architectureof branchedpolymers. Characterize comb polymers.



8.Structural architectureof branchedpolymers.Characterize hyperbranched polymers.
Polymers are inorganic and organic, amorphous and crystalline substances, consisting of "monomeric links", connected in long macromolecules by chemical or coordination bonds. Polymers are substances with a molecular mass of several thousand to several million.
Branched polymers consist of macromolecules, the main chain of which, unlike linear, contains arbitrarily located lateral branches of several atoms to the size of the main chain. The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center. Branched polymers containing branched branches in each link, for example polyhexadecyl acrylate, also include branched polymers:

In the structure of the polymer, a monomer unit can be distinguished-a repeating structural fragment comprising several atoms.
The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center.
Hyperbranched polymers (HPs) are highly branched three-dimensional (3D) macromolecules. Their globular and dendritic architectures endow them with unique structures and properties such as abundant functional groups, intramolecular cavities, low viscosity, and high solubility.

Hyperbranched polymers can be prepared by means of single-monomer methodology (SMM) and double-monomer methodology (DMM). In SMM, the polymerization of an ABn or latent ABn monomer leads to hyperbranched macromolecules. SMM consists of at least four components: (1) polycondensation of ABn monomers; (2) self-condensing vinyl polymerization; (3) self-condensing ring-opening polymerization; (4) proton-transfer polymerization. In DMM, direct polymerization of two suitable monomers or a monomer pair gives rise to hyperbranched polymers. A classical example of DMM, the polymerization of A2 and Bn (n>2) monomers, is well known.
9. Structural architectureof branchedpolymers.Characterize dendrimers.
Polymers are inorganic and organic, amorphous and crystalline substances, consisting of "monomeric links", connected in long macromolecules by chemical or coordination bonds. Polymers are substances with a molecular mass of several thousand to several million.
Branched polymers consist of macromolecules, the main chain of which, unlike linear, contains arbitrarily located lateral branches of several atoms to the size of the main chain. The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center. Branched polymers containing branched branches in each link, for example polyhexadecyl acrylate, also include branched polymers:

In the structure of the polymer, a monomer unit can be distinguished-a repeating structural fragment comprising several atoms.
The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center.
Dendrimers (from the Greek word tree)

represent a new class of polymers with regularly branched structure. Dendritic molecules are characterized by structural perfection. Dendrimers and dendrons are monodisperse and usually highly symmetric, spherical compounds. The field of dendritic molecules can be roughly divided into low-molecular weight and high-molecular weight species. The first category includes dendrimers and dendrons, and the latter includes dendronized polymers, hyperbranched polymers, and the polymer brush
The rheological behavior of branched polymers contrasted with that linear melta motivates discussion of a molecular model of a melt of H-polymers. Concentrating on the dynamics of the ‘backbone’ we derive and discuss the linear viscoelastic properties in the light of experiments by Roovers,
10. Structural architectureof branchedpolymers.Characterize starpolymers.
Polymers are inorganic and organic, amorphous and crystalline substances, consisting of "monomeric links", connected in long macromolecules by chemical or coordination bonds. Polymers are substances with a molecular mass of several thousand to several million.
Branched polymers consist of macromolecules, the main chain of which, unlike linear, contains arbitrarily located lateral branches of several atoms to the size of the main chain. The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center. Branched polymers containing branched branches in each link, for example polyhexadecyl acrylate, also include branched polymers:

In the structure of the polymer, a monomer unit can be distinguished-a repeating structural fragment comprising several atoms.
The limiting case of branched polymers is a star-shaped, whose macromolecules are a set of chains emerging from one center.

A - linear polymer; Б, B, Г – branched polymers; Д, E – cross-linked polymers; E – ladder polymer.
11. Evaluate the differences between molecular structure of low and high molecular compounds. Give examples.
The macromolecules of polymers, in contrast to molecules of low molecular weight substances, are non-volatile, they are characterized by lower diffusion rates, and polymer solutions are characterized by smaller values of colligative properties compared with solutions of low-molecular compounds. However, the most significant and fundamental differences in the properties of high- and low-molecular compounds arise only when a large molecular mass is combined with the chain structure of macromolecules. Almost all polymers produced by industry and natural polymers of organic origin are chain-like. This means that the length of the macromolecule is much larger than its transverse dimension. This can be easily estimated if we assume that the molecules of the monomers from which the macromolecules are formed have a shape close to spherical. In this case, the transverse dimension of the macromolecular chain is equal to the diameter of the monomer molecule l, and the length of the elongated chain L, called the contour line, is equal to: L=nl, where n is the number of monomeric units in the chain, which is equal to the ratio of molecular of polymer and monomer. The most valuable properties of chain polymers are fully manifested at M> 105. Typical monomers, of which large-tonnage polymers are obtained, have a molecular weight of the order of 102. It follows that the characteristic feature of macromolecules of chain polymers is: (L/l)  103 . This property is realized in such polymeric materials as rubber. The second implies the manifestation by polymers of the properties inherent in a solid and liquid, ie, a combination of reversible and irreversible (flow) deformations. Viscoelasticity leads to plasticity and reduced brittleness of polymer materials called plastics, since the irreversible movement of macromolecules under load causes stress relaxation and prevents material from destruction. Polymer solutions are characterized by swelling, in which the volume the soluble polymer can increase by an order of magnitude or more, a large viscosity and ability to gel formation. Often, 1 to 2% of a solute, for example gelatin, is sufficient to make the solution lose its fluidity. The chemical behavior of macromolecules is also related to their chain structure. This circumstance predetermines the increased probability of cooperative processes,
103 . This property is realized in such polymeric materials as rubber. The second implies the manifestation by polymers of the properties inherent in a solid and liquid, ie, a combination of reversible and irreversible (flow) deformations. Viscoelasticity leads to plasticity and reduced brittleness of polymer materials called plastics, since the irreversible movement of macromolecules under load causes stress relaxation and prevents material from destruction. Polymer solutions are characterized by swelling, in which the volume the soluble polymer can increase by an order of magnitude or more, a large viscosity and ability to gel formation. Often, 1 to 2% of a solute, for example gelatin, is sufficient to make the solution lose its fluidity. The chemical behavior of macromolecules is also related to their chain structure. This circumstance predetermines the increased probability of cooperative processes,
Дата добавления: 2018-04-04; просмотров: 437; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!
