Description of a Radioactive Source
What is Radioactivity?
1. Ionizing radiation. 2. Radioactive elements. 3. The nature of radioactivity. 4. Alpha radiation. 5. Beta and Gamma radiation. 6. What is an isotope? 7. The radiation series. 8. The energy of radiation.8.1 Description of radioactive sources. 8.2. Alpha (α) radiation. 8.3. Beta (β) radiation. 8.4. Gamma (γ) radiation. 9.The penetration of radiation. 10. Ionization. Excitation.
Ionizing radiation
Living organisms as well as non-living objects of the environment are affected by set of various factors of the physical nature including radiation (visible light, ultraviolet, infra-red, magnetic fields, radio-waves of various ranges). Ionizing radiation of natural radioactive elements and isotopes as well as space radiation also acts.
The common name for both radiation from x-ray machines and radioactive sources is ionizing radiation. The name indicates that the radiation has sufficient energy to ionize atoms and molecules. Ionization takes place when an electron is removed from its position in the atom or molecule. Since a molecule usually has no net charge to begin with, the loss of a negative electron leaves behind a positive ion. The electron can then end up on another molecule which then becomes a negative ion. The creation of positive and negative ions in matter is the signature of radiation, allowing us to detect and categorize it. Ionizing radiation is distinct from low energy radiations which include ultraviolet, visible, infrared, microwaves and radio waves that produce effects, for the most part, of a different nature.


 2. Radioactive Elements
2. Radioactive Elements
The atomic structure of most elements contains a nucleus that is stable. Under normal conditions, these elements remain unchanged indefinitely. They are not radioactive. Radioactive elements, in contrast, contain a nucleus that is unstable. The unstable nucleus is actually in an excited state that can not be sustained indefinitely; it must relax, or decay, to a more stable configuration. Decay occurs spontaneously and transforms the nucleus from a high energy configuration to one that is lower in energy. This can only happen if the nucleus gives off energy. The energy emitted by the relaxing nucleus is radiation. All radioactive elements have unstable nuclei; that is what makes them radioactive.
|
|
|
Thus, the radioactivity is considered as a spontaneous or artificial transformation of nucleus of atom of unstable isotopes of a chemical element from the basic state in other isotope of this or other element that is accompanied by emitting of energy of elementary particles or nucleus.
The Nature of Radiation
The energy emitted by an unstable nucleus comes packaged in very specific forms. In the years that followed the discovery of radioactivity, determining the kind of radiation emitted from radioactive compounds was of great interest. It was found that these radiations consisted of three types called: alpha (α), beta (β) and gamma (γ) radiations after the first three letters in the Greek alphabet (see Figure 3.1).
The nuclear emission transforms the element into either a new element or a different isotope of the same element. A given radioactive nucleus does this just once. The process is called decay or disintegration.
The law of radioactive decay asserts that the identical portion of available nucleus disintegrates per unit time. The measure of the number of disintegrations per unit time is called the decay rate. The decay rate is proportional to the number of radioactive atoms present.

FIG 3-1.
A radioactive atom possesses an unstable nucleus. This means that radioactive atoms will emit radiation sooner or later and convert into a more stable state. The types of radiation that may be emitted are called alpha (α), beta (β) and gamma (γ) radiation.
The evidence for the three types of radiation comes from an experiment in which the radiation from radioactive compounds was passed through a magnetic field. γ-rays passed through the field without disturbance, whereas the two other types were deflected from a straight line. Because it was known at that time that charged particles are deflected when they pass through a magnetic field, the conclusion was evident; γ-rays have no charge while α- and β-radiations consist of charged particles. The α-particles, deflected in one direction are positive whereas the β-particles, deflected in the opposite direction, are negative.
|
|
|
Alpha radiation
In 1903, Ernest Rutherford (a New Zealander who worked in Cambridge, England most of his life) performed a simple and elegant experiment showing that the α-particle is the nucleus of the helium atom. Rutherford positioned one glass tube inside a second glass tube. The inner tube contained a radioactive source that emitted α-particles. The outer tube contained a vacuum and at each end there was an electrode. The α-particles passed through a thin window, picking up two electrons on the way, and entered the outer tube as a gas. When Rutherford turned on the high voltage between the electrodes, the tube emitted light at very specific wavelengths (specific colors). He compared wavelengths of this light with the wavelengths of light produced by a similar tube that he had filled with helium gas. The colors of the light were identical. Rutherford concluded that α-particle is simply the nucleus of a helium atom and that when the α-particles reach the outermost tube they have picked up two electrons to become helium atoms.
|
|
Beta and gamma radiation
Experiments have shown that the β-particle is a fast moving electron, whereas γ-radiation is an electromagnetic wave. Other examples of electromagnetic radiation are ultraviolet (UV), visible light, infrared and radio waves. Electromagnetic radiation is characterized by its wavelength or frequency. The wavelength is the distance from one wave peak to the next and the frequency is the number of waves passing a given point per second. Through quantum mechanics it is known that particles can be described as waves and vice versa. Thus, γ-rays and other electromagnetic radiation are sometimes described as particles and are called photons.
What is an isotope?

In several places throughout this book isotopes are mentioned. Some isotopes are unstable, and therefore radioactive, while others are stable, and thus nonradioactive. What is an isotope? An element can exist in several versions that are chemically equivalent but have different atomic weights. The atomic weight of an element can be changed by altering the number of neutrons in the nucleus.
|
|
|
This is illustrated above for the most common of the elements, hydrogen.
The nucleus of an atom consists of protons and neutrons (called nucleons). The number of protons determines the element and the number of nucleons determines the atomic weight. Isotopes are atoms with the same number of protons, but with different numbers of neutrons.
Isotopes are written using the symbol for the element, such as H for hydrogen, O for oxygen, and U for uranium. Furthermore, the nucleon number is used to separate the isotopes. For example the three hydrogen isotopes are written as H-1, H-2 and H-3 (you will often see the isotopes written as 1H, 2H and 3H).
Since the hydrogen isotopes are so well known, they have attained their own names. H-2 is called deuterium and H-3 is called tritium. When tritium disintegrates it emits β-particle with an average energy of only 5.68 keV and maximum energy of 18.6 keV (1 ke V equals 1000 electron volts of energy).
In nature, 99.985% of hydrogen is the H-1 isotope. In ordinary water, only one out of 7000 atoms is deuterium. Due to nuclear processes in the atmosphere there are small amounts of tritium. Tritium is widely used in research.
Potassium is another example of an element that has radioactive isotopes. Potassium consists of 93.10% K-39, 6.88% K-41 and 0.0118% of the radioactive isotope K-40. The latter isotope is present because it has a very long half-life of 1.27 billion years. The Earth's crust contains a lot of potassium. In spite of the small fraction of K-40, the radiation from this isotope is quite important. All living organisms contain some radioactive potassium. For example a human being contains, on average, about 60 Bq/kg body weight of K-40.
The Radioactive Series
A radioactive atom is unstable and will eventually eject a particle and/or a photon to attain a more stable state. Certain atoms are still unstable even if radiation has been emitted. Uranium is a typical example shown in Figure 3.2.
|
|
|
| Type of radiation | Isotope | Half-life | ||
| α | ○ Uranium-238 | 4.47 | billion years | |
| β | ○ Thorium-234 | 24.1 | days | |
| β | ○Protactinium-234 | 1.17 | minutes | |
| α | ○Uranium-234 | 245,000 | years | |
| α | ○Thorium-230 | 77,000 | years | |
| α | ○Radium-226 | 1600 | years | |
| α | ● Radon-222 | 3.82 | days | |
| α | ● Polonium-218 | 3.05 | minutes | |
| β | ● Lead-214 | 26.8 | minutes | |
| β | ● Bismuth-214 | 19.8 | minutes | |
| α | ○ Poionium-214 | 0.164 | milliseconds | |
| β | ○ Lead-210 | 22.3 | years | |
| β | ○ Bismuth-210 | 5.01 | days | |
| α | ○Polonium-210 | 138.4 | days | |
| ○ Lead-206 | Stable | |||
FIG 3-2.
Uranium-radium-series. The start of the series is U-238 and the end point is Pb-206. The first isotope has the longest half-life, 4.47 billion years. Radon and the radon decay are encircled (notice the short half-lives).
The chemical symbol for uranium is U. The start of this decay series is the isotope U-238 or 238U. When this isotope emits α-particle, it is changed into thorium-234.
Th-234 is also unstable and it emits β-particle, forming a new decay product, protactinium-234. The new product is still not stable and the decay processes continue step by step until Pb-206 is reached. Altogether, 14 disintegrations take place before U-238 ends up as a stable lead isotope (the whole series is shown in Figure 3.2). A series of unstable atoms where one atom changes into another is called a radioactive family or simply a radioactive series. Altogether, there are 4 naturally occurring radioactive families on Earth. Two of these have almost disappeared and only the uranium-radium series and thorium series are still active.
A radioactive source consists of a large number of unstable atoms. For example, one gram of the iodine isotope I-131 consists of 4.6 • 1021 atoms. All these atoms will sooner or later emit radiation, but these emissions do not take place simultaneously. It is a statistical process, with one atom decaying every now and then. When one half of the atoms have decayed the source has gone through what is called one "half-life". Not all atoms have decayed after two half-lives, ¼of the unstable atoms remain.
This uranium-radium series has been present from the beginning of the Earth. The first step in this series has a very long half-life of almost 5 billion years, the present age of the Earth. Thus, we are only now into the second half-life of the uranium-radium series. The two radioactive series that have almost disappeared have done so because the half-lives are much shorter.
The Energy of the Radiation
In order to detect radioactivity and to evaluate the biological effect of the radiation it is important to have information about the energy as well as the type of radiation emitted. The unit used for energy is the electron volt (abbreviated eV). By definition, an electron volt is the energy attained by an electron when it is accelerated through a voltage gap of 1 volt. The product of voltage and the electron charge (given in Coulombs, C) gives the relation between electron volt and a unit of energy, the joule (J):
1 eV = 1 V · 1.6 · 10-19 C = 1.6 · 10-19 J.
The electron volt is a very small unit. The energy usually set free by disintegration varies from a few thousand electron volts (keV) to approximately 6 million electron volts (MeV).
Description of a Radioactive Source
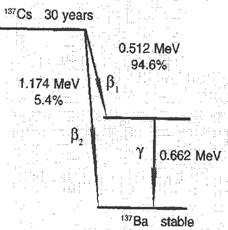
How is a radioactive source described? The intensity of the source depends on the number of atoms that disintegrate per second (i.e. the number of becquerels). Other parameters are: type of radiation, half-life (the half-life period is the time during which the quantity of atoms of a radioactive isotope decreases twice), and energy of the radiation. All these parameters can be given by a decay scheme. For example, the radioactive isotope Cs-137, which is the most important radioactive waste product from a nuclear reactor, has the decay scheme given in Figure 3.3.
|
|
A decay scheme is another way physicists use to convey information. The scheme tells us about the types of radiation emitted, the energy involved, half-life, etc. This type of information is necessary in calculating radiation doses and risks.
FIG. 3-3.
A scheme for the disintegration of Cs-137. The state of the nucleus is given by horizontal lines. The atomic number increases left-to-right, Cs is 55 and Ba is 56. The vertical scale is the energy of the nucleus, given in MeV. The vertical distances between the lines indicate the energy difference. This energy is set free by disintegration, appearing as β-particle or γ-ray.
The decay scheme shows that Cs-137 is transformed into the stable barium isotope Ba-137. This can take place via two different routes:
1. In 94.6% of the disintegrations a β-particle is emitted with an energy of 0.512 MeV (106 eV), followed immediately by a γ-ray with an energy of 0.662 MeV.
2. In 5.4% of the disintegrations the stable barium isotope is reached directly by emitting only a β-particle, with energy of 1.174 MeV.
The decay scheme also shows that the half-life of Cs-137 is 30 years. In addition, one might guess that Cs-137 can be observed by measuring the emitted γ-rays. γ-rays are easy to detect because they are very penetrating, a quality that is described at the end of this chapter.
8.2. 
 Alpha Radiation
Alpha Radiation
The energy of α-particle, when it is emitted by a nucleus, is usually a few MeV. Some of the properties which are characteristic of α-particles are:
• The α-particles from one particular radioactive source have the same energy. For example α- particles from U-238 always have a starting energy of 4.19 MeV.
• When α-particle passes through a material, іt rapidly loses energy through numerous collisions with the electrons that make up the atoms and molecules. Because the collisions produce ionizations, a high density of ions is deposited in the material tracing out a linear track. The energy of the α-particle is ultimately dissipated by this large number of low energy interactions and it stops at the end of the track.
The energy deposited per unit length of the track is called the linear energy transfer (abbreviated LET). An example is given in Figure 3.4. The range of α-particle from a radioactive source is very short in animal tissue and in air the range is only a few centimeters. As can be seen from Figure 3.4 the energy loss along the track is not constant but gradually increases toward the end of the track.
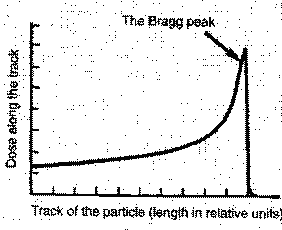
The use of heavy particle beams offers potential advantages in cancer therapy. If the "Bragg peak" lands on the tumor, the tumor dose is larger than the dose to the surrounding healthy tissue. The goal is to maximize damage to the tumor while minimizing damage to healthy tissue.
FIG. 3-4.
The energy deposition along the track of α -particle.
Beta Radiation
The energy of a β-particle (a fast electron or positron - the latter is a positively charged electron) is usually much smaller than the energy of α-particles. Furthermore, the energy of the β-particles varies from one disintegration to another; β-particle emission from a source is described by a spectrum of energies. Usually, the maximum energy is given in the decay schemes such as that shown earlier in Figure 3.3.
Consider this in more detail. In disintegration the nucleus changes from one energy state to another. This change is given as a well defined energy gap. However, the β-particles do not all have the same energy. The explanation is that, together with the β-particle, a tiny neutrally charged particle is emitted. This particle was called a neutrino by the Italian physicist Enrico Fermi. (The term neutrino means "the small neutral particle".) The sum of the energies of the electron and the neutrino is equal to the energy gap in the decay scheme.
The rule of thumbdetermines the average β-particle energy:The β-particle energy for a source varies from zero up to a maximum. The average energy is approximately 1/3 of the maximum energy.
β-particles are stopped by collisions with electrons in materials in a process similar to the way α-particles are stopped. According the rule of thumb one can say that β -particle with energy 1 MeV will have a range in water or soft tissue of 0.5 cm. The β-particles from Cs-137 have an average energy of 0.2 MeV. If these particles hit the skin, the penetration into the body would be less than 1 mm. However, if a sufficient number of these β-particles hit the skin, it will be burned.
Gamma Radiation
The energy of a γ-ray is given by the expression:
| E = hν |
 where h is a fundamental constant known as Planck's constant and ν is the frequency of the radiation wave. The radiation can be considered to consist of small energy packages called quanta or photons. The energy of the γ-ray ranges from 0.1 to l.5 MeV. The cesium isotope Cs-137 emits γ-rays with energy of 0.662 MeV. The cobalt isotope Co-60 emits two quanta with energies of 1.17 and 1.33 MeV.
where h is a fundamental constant known as Planck's constant and ν is the frequency of the radiation wave. The radiation can be considered to consist of small energy packages called quanta or photons. The energy of the γ-ray ranges from 0.1 to l.5 MeV. The cesium isotope Cs-137 emits γ-rays with energy of 0.662 MeV. The cobalt isotope Co-60 emits two quanta with energies of 1.17 and 1.33 MeV.
Gamma-rays and x-rays are absorbed differently from α-particles. When γ-rays penetrate a material, the intensity of the radiation (I) decreases according to an exponential formula:
| I (x) = Io ·e-μx |
where x is the depth in the material and μ is the absorption coefficient (μ describes how the radiation decreases per unit length for each type of material). The absorption coefficient has three different components. This is because three processes are involved: photoelectric effect, Compton scattering (inelastic scattering) and pair production.
Photoelectric effectis a process in which a photon interacts with a bound electron. The photon itself disappears, transferring all its energy to the electron and thereby imparting kinetic energy to the electron. This is the most important absorption process for radiation with energy less than about 100 keV (which is the type of radiation used in medical diagnostics). The photoelectric effect varies dramatically with the electron density of the absorbing medium. Thus material that contains atoms with high atomic numbers, e.g., the calcium in bone, gives strong absorption due to the photoelectric effect.
Compton scatteringis a process in which a photon collides with a bound electron and where the photon energy is considerably greater than the electron binding energy (see Figure 3.5).
After the interaction, the photon continues in a new direction with reduced energy and the electron attains enough energy to leave the atom. We call this electron a secondary electron. The Compton process is the most important absorption process for photons with energies from about 100 keV to approximately 10 MeV (the type of radiation mainly used for radiation therapy).
Pair productionis a process in which the energy of the photon is used to produce a positron-electron pair. The photon energy must be above 1.02 MeV, the threshold energy for forming two electrons. The process takes place near the atomic nucleus and is the most significant absorption mechanism when the photon energy is above about 10 MeV.
 FIG. 3-5.
FIG. 3-5.
The drawing describes the process. The incoming photon interacts with an electron and the result is that the photon is scattered and its energy is reduced. The electron is ejected and becomes a "secondary electron".
The Penetration of Radiation
When using a gun, the penetration by the bullet depends on the energy of the bullet as well as the composition of the target. For example, a pellet from an air gun will be stopped by a few millimeters of wood but a bullet from a high powered rifle will pass through many millimeters of steel. It is similar with ionizing radiation. There are large differences in penetrating ability depending on the type of radiation (α-, β- or γ-radiation).
Alpha-particlesfrom radioactive sources have energies up to 6 or 7 MeV with a range in air of only a few cm. In condensed matter the range is much shorter; α-particles will not even penetrate clothing. As long as the α-particle source is outside the body, there is no danger. If, however, the source is inside the body, all the energy is deposited in the body. It is mainly the heavy elements such as uranium, radon and plutonium which are α-emitters.
Beta-particleswith energy of 1 MeV have a range in soft tissue of approximately 5 mm. The majority of β-particles have energy far less than 1 MeV. Consequently, almost all β-particles coming from sources in the environment are stopped by clothing.
Gamma radiationhas the ability to penetrate tissue and even concrete. For example, 50% of the γ-rays from Cs-137, with energies of 0.662 MeV will penetrate a water layer of about 9 cm. We call thisa half-value laуеr. Five half-value layers (less than 0.5 meter of water) will reduce the radiation by 97%. γ-radiation is easy to measure, whether the source is outside or inside the body. Consequently, isotopes emitting γ-radiation are used in medical diagnoses.
X-rays and γ-rays will easily penetrate the human body. This property is utilized when x- and γ-rays are used for diagnostic purposes, α- and β-particles, on the other hand, lose their energy within a short distance and cannot penetrate the body. Because of these penetration properties, γ-radiation is easy to observe whereas α-and β-radiation are more difficult to detect. Thus, special instruments are often needed in order to observe α-and β-rays. The following conclusions can be drawn:
§ If a radioactive source is on the ground, such as in a rock, the α- and β-radiation will be stopped by air and clothes. Only γ-rays would penetrate into the body and deliver a radiation dose.
§ When a radioactive source is insidethe body, it is a different situation. α-and β-particles are completely absorbed within a short distance in the tissues, whereas only a certain fraction of the γ-radiation is absorbed. The rest of the γ-radiation escapes and can be observed with counters outside the body. Consequently, if you eat food containing radioactive compounds, they can be easily measured if γ-rays are emitted.
It is possible then to measure the radioactivity that is inside animals and humans who have eaten food containing Cs-137 due for example to fallout from nuclear tests or nuclear accidents. For adults, approximately 50% of the γ-radiation escapes the body and the other half is absorbed by the body. Other important isotopes such as Sr-90 (strontium) and Pu-239 (plutonium) are very difficult to observe since they only emit β-particles and α-particles.
Ionization. Excitation.
Ionizing radiation is a more precise name for all types of radiation with energy large enough to ionize a molecule (below, M = molecule). Included under this designation are types of radiation from radioactive sources (α-, β- and γ-rays), x-rays, short wave length UV, particles from accelerators, particles from outer space, and neutrons.

Most atoms and molecules have ionization energy of 10eV and more. Certain molecules in liquids and іn the solid state may have ionization energy as low as 6 eV. This means that UV-radiation with a wavelength below approximately 200 nm (6.2 eV) may cause ionization. Radiation with energy of 1 MeV has enough energy to yield about 150,000 ionizations if all the energy deposited produces ions.
The electron which is ejected from the molecule in an ionization process is called asecondary electron. Secondary electrons with a starting energy of 100 eV or more make their own tracks and will ionize and excite other molecules. These electrons are called delta rays.
Ionizing radiations not only ionize but can also excite molecules. Excitations are also produced by long wavelength UV and visible light (called non-ionizing radiation). An excitation occurs when the molecule attains extra energy. This is done by increasing the vibrational, rotational, or electronic energies of the molecule. These excited states have short life times (less than milliseconds) and sometimes relax back to the ground state by emitting light. Light emission from an excited molecule, called fluorescence and phosphorescence, is a property that is used to measure and characterize ionizing radiation.
Excitedand ionizedmolecules are very reactive and have short life times. These reactive products represent the starting point for all radiobiological effects, such as cancer. The biological effect increases with the number of ions and excited molecules formed.
Control points to Charter 3
1) What is ionizing radiation?
2) Radioactivity.
3) The law of radioactive decay.
4) α-particles and Alpha radiation.
5) What is the difference between Beta and Gamma radiation?
6) What is an isotope?
7) Radioactive series; half-lives of radioactive series.
8) The unit for radiation energy. Radioactive source.
9) Alpha radiation. LET.
10) Beta radiation. The rule of thumb.
11) Gamma radiation. Photoelectric effect. Compton scattering. Pair production.
12) Penetration of radiation (α-particles, β-particles, γ-particles).
13) What is the difference between ionization and excitation?
Science vocabulary
1.disintegration розпадання; подрібнення на складові частини
2. atomic weightатомна маса
3.nucleon нуклон
4. attain досягати
5.deuterium дейтерій, важкий водень
6.eject вивергати; викидати
7. radioactive series радіоактивний ряд
8. penetrate проникати всередину
9.dissipate розсіювати(ся)
10.radioactive decayрадіоактивний розпад
11. trace out креслити, накреслювати
12. linear trackлінійний трек
13. energy gapперепад енергії; енергетичний інтервал
14.tinyдуже маленький, крихітний
15.rule of thumb практичний метод; емпіричне правило
16.collision зіткнення
17. quantum (a) квант
18.photoelectric effect фотоелектричний ефект
19.inelastic scattering непружнерозсіювання
20.pair production утворення (електронно-позитронних) пар
21. impart надавати; передавати
22. secondary electronвторинний електрон
23.pellet кулька
24.half-value layerшар половинного послаблення (іонізуючого випромінювання)
25.nm (nanometre) нанометр
26. fluorescence флуоресценція, свічення
27. phosphorescence фосфоресценція, свічення
Дата добавления: 2018-02-15; просмотров: 370; Мы поможем в написании вашей работы! |

Мы поможем в написании ваших работ!